- [Interview] Kim Ji-heon, Head of Research and Development at Bukwang 바카라 사이트 추천디시
- Leveraging 바카라 사이트 추천디시D and BD strengths, advancing 바카라 사이트 추천디시D with an ‘investment-oriented’ approach
- Post-OCI acquisition restructuring, reorganizing novel drug candidates
- Introducing ‘first generics’ to drive sales, with five product launches planned by 2026
- Positioning as a CNS specialist, accelerating development of a one-month long-acting ‘Latuda’ injection
- ‘Global open innovation’ at the core of Bukwang 바카라 사이트 추천디시's DNA, driving competitiveness through platform integration

[by Yu, Suin] "'Global open innovation' has long served as the hallmark and strength of Bukwang Pharmaceutical's research and development (R&D). Now we see the moment as one to 'internalize' this platform, and the prospects appear favorable."
Kim Ji-heon, Executive Director and Head of Bukwang 바카라 사이트 추천디시's Research and Development Division, stated in a recent interview with <THE BIO> that he is committed to delivering tangible outcomes in both 'structural improvement' and 'R&D' beginning this year.
Kim is regarded as a rare figure in the industry, capable of bridging both R&D and business development (BD). This dual perspective allows him to view R&D through the lens of 'investment.’ From a researcher's perspective, R&D can often translate into limitless investment, whereas the business side demands a more objective judgment. Even when evaluating the same asset, researchers tend to prioritize its 'potential,' while business professionals focus on valuation and profitability.
Kim explained that his efforts have centered on restructuring Bukwang Pharmaceutical's pipeline and business framework to ‘improve its fundamentals’ and ‘generate profits.’ "From a pipeline perspective, we have begun to invest in R&D with greater selectivity and discipline," he noted. "We are also carefully evaluating whether to pursue additional investments in our subsidiaries."
◇Excessive focus on 'Global R&D' restrains business performance…"Need for internalization"
Kim assumed his position at Bukwang 바카라 사이트 추천디시 in September 2023. He entered the 바카라 사이트 추천디시 industry after earning a master's degree in pharmacy from Chung-Ang University and completing an executive-level business administration (EMBA) program at Aalto University in Finland. Over the past 25 years, he has built a career in numerous Korean and international 바카라 사이트 추천디시 companies, including Chong Kun Dang, Roche Korea, Eisai Korea, and GC Biopharma, gaining extensive expertise in R&D, business development, licensing, regulatory affairs, and investment.
Kim underscored that Bukwang 바카라 사이트 추천디시's has traditionally concentrated heavily on R&D but now requires a structural shift to better support business performance. Although the company has long pursued R&D growth through a global open innovation strategy, it has not sufficiently internalized its platform, an approach that has yielded only limited results.
Bukwang 바카라 사이트 추천디시 has engaged in collaborations with Korean and international biotech ventures through diverse approaches, including mergers and acquisitions (M&A), equity participation, joint ventures (JVs), and joint R&D initiatives. At one point, the company invested roughly KRW 7.5 billion (approximately USD 5.4 million) in six biotech ventures in the United States, Canada, and Korea, ultimately generating more than KRW 100 billion in profits, an outcome that underscored the effectiveness of its global open innovation strategy. Contera Pharma, its Danish subsidiary, serves as a representative case, having pursued global new drug development under the open innovation model since Bukwang 바카라 사이트 추천디시 acquired a stake in the company in 2014.
"Bukwang Pharmaceutical's R&D strategy is without precedent. It would not be surprising for large pharmaceutical companies like GC Biopharma, Daewoong Pharmaceutical, or Yuhan Corporation to pursue open innovation. What is remarkable, however, is that a company with annual sales of less than KRW 200 billion set its sights on the global market, rather than limiting itself to the local market, at such an early stage," Kim remarked.
"The strength of this 'global R&D strategy' lies in the fact that, once it gains traction, it can deliver significant success. Yet the heavy emphasis on global open innovation inevitably came at the expense of investment in our in-house research center," he further explained. "In the midst of this, the company faced financial strain and was even forced to suspend clinical trials for the subsidiary pipeline (Phase 1 for 'SOL-804' and Phase 2 for 'JM-010'). This, however, became an inflection point, prompting the establishment of clearer standards for both R&D and subsidiary management."
The role of OCI, Bukwang Pharmaceutical's largest shareholder, was also a driving factor behind this decision. OCI Group, active in the solar and chemical industries, acquired Bukwang Pharmaceutical in 2022 with the aim of fostering biotechnology as a new growth engine. At the time, the move drew considerable industry concern over potential conflicts of interest and limited synergy arising from the convergence of disparate industries. However, Kim emphasized that the rigorous investment verification process and a streamlined decision-making structure under OCI’s ownership accelerated Bukwang Pharmaceutical's structural reforms and strengthened the efficiency of its investment management.
"OCI's support created an opportunity for Bukwang Pharmaceutical to move away from old practices, reassess its processes from a fresh perspective, and overcome organizational inertia," Kim noted. "In addition, the rapid decision-making structure typical of chemical companies has accelerated the pace of investment."
◇Pipeline restructuring at the forefront…Strengthening the R&D workforce and expanding the CNS portfolio
Kim concentrated on reshaping Bukwang 바카라 사이트 추천디시's pipeline and business framework with the goal of strengthening structural efficiency and generating profits. Among these initiatives was the decision to return the rights to the diabetes treatment candidate MLR-1023. In 2024, Bukwang 바카라 사이트 추천디시 relinquished the Asian rights, excluding Japan, to its U.S.-based partner Melior 바카라 사이트 추천디시s and additionally transferred the manufacturing patents related to MLR-1023 to the company.
"MLR-1023 is an asset we have held since 2013. The Phase 2b clinical trial results were disappointing, and we concluded that, rather than retaining it, it would be more meaningful to transfer it to another party to create value," Kim recalled.
"From a pipeline standpoint, we have adopted a more rigorous and selective approach to R&D investment. At the same time, we are carefully evaluating whether to pursue further investments in our subsidiaries," he further commented.
He also reorganized the company to reinforce its domestic development capabilities. Convinced that securing new products is critical for accelerated growth, he expanded the product portfolio through license transfers and acquisitions while simultaneously advancing the development of improved new drugs through in-house R&D. Reflecting this effort, the number of researchers at Bukwang 바카라 사이트 추천디시's laboratory rose from 42 at the end of last year to 48 by the end of the first half of this year.
In H2 2024, the company created a dedicated sales unit, the ‘CNS Business Division,’ to strengthen its presence in the central nervous system (CNS) field. This division concentrated on CNS-related products and spearheaded the market launch of Latuda (lurasidone hydrochloride), a novel antipsychotic drug.
Latuda, an atypical antipsychotic developed by Japan’s Sumitomo Pharma, is approved for the treatment of schizophrenia and bipolar I depression. Bukwang Pharmaceutical holds exclusive development and sales rights for the Korean market, where the drug was launched with reimbursement coverage in August of last year. In May, monthly prescription sales surpassed KRW 1 billion (approximately USD 721 thousand) for the first time.
In March, the company expanded its CNS portfolio with the reimbursement launch of ‘Ariplus Tablets’ (donepezil hydrochloride hydrate + memantine hydrochloride), an Alzheimer's disease treatment. The addition helped drive Bukwang Pharmaceutical's performance in the first half of the year, with sales reaching KRW 90.4 billion, operating profit KRW 5.1 billion, and net profit KRW 6.3 billion. Compared with the same period last year, sales rose 26.8%, while both operating profit and net profit turned to surplus.
◇Driving sales next year with the ‘first generic’ launch…establishing ‘long-acting injectables’ as a new growth engine
With organizational restructuring at Bukwang Pharmaceutical now complete, Kim intends to accelerate the development of new growth drivers starting this year. To bolster domestic business expansion, the company plans to introduce at least five new products, including ‘first generics,’ between this year and early 2026. In addition, new research projects, such as incrementally modified drugs, have been initiated as part of his mid- to long-term strategy. Over the long term, Bukwang Pharmaceutical, together with its subsidiary Contera Pharma, is pursuing the development of innovative therapeutics.
"Bukwang Pharmaceutical is not a typical novel drug development venture. As a 'pharmaceutical company,' our responsibility is to develop and market medicines, generate revenue from them, and contribute to public health," Kim remarked. "Given the shortage of new products Bukwang Pharmaceutical has faced in recent years, it was imperative to secure differentiated offerings sooner rather than later. Establishing a balanced portfolio of short-, mid-, and long-term growth drivers is essential to sustaining the company's growth."
"To accelerate product launches, we have expanded our portfolio by transferring or acquiring licensing rights from external companies, such as in the case of Ariplus, as well as by acquiring technologies and research projects," he further explained. "Including products already in the market, the pipeline scheduled for release by early 2026 spans the CNS, diabetes, and liver sectors. In particular, we plan to launch a CNS product containing an ingredient not previously marketed in Korea around February next year. As the first generic of its kind, we anticipate it will make a substantial contribution to sales."
Kim noted that the company has expanded its focus to novel drug research by reinforcing the capabilities of its research institute. In the first half of this year alone, Bukwang Pharmaceutical initiated four research projects targeting incrementally modified drugs and generics, while also making notable progress in the development of a ‘long-acting injectable platform,’ a specialized form of incrementally modified drug.
The long-acting injectable platform has emerged as a core project that Bukwang Pharmaceutical is actively pursuing. Kim, who previously conducted research on sustained-release microsphere technology at a Korean pharmaceutical company, now seeks to apply this platform to Latuda, one of the company’s newly established growth drivers. To this end, he is pursuing a three-pronged approach, including joint research, contract research, and in-house research, areas in which progress has already been made.
"We have recently verified the feasibility of clinical trials and are now in the follow-up stage. Nevertheless, recognizing there are areas we cannot address independently, we are also seeking collaborations with major Korean biotechnology firms," Kim stated.
"With open innovation embedded in its DNA, our company is working to maximize both its internal capabilities and external collaborations in order to deliver rapid results," he added.
"Long-acting injectables hold clear potential to improve patient compliance and maximize therapeutic effects in conditions like schizophrenia, with their value and growth prospects already demonstrated in the global market," Kim further said. "Moreover, as no products incorporating the Latuda ingredient currently exist, we believe this offers strong market competitiveness. Our objective is to develop a long-acting injectable with a duration of at least one month."
Kim stressed that securing platform technology is critical for Bukwang Pharmaceutical to move to the next stage. "The success stories of Korean and international pharmaceutical and biotech companies show a common pattern: each possessed unique platforms or core competencies that enabled them to generate lasting value. When proven platforms were combined with open innovation, the outcomes were even more significant," he explained.
"Bukwang Pharmaceutical has global open innovation in its DNA, and we are executing it effectively. What we lack is a platform," Kim further remarked. "Our open innovation capabilities are well established, and I believe that securing a proprietary platform will give us a strong chance of success. My goal, and the company’s direction, is to combine these two elements to maximize value."
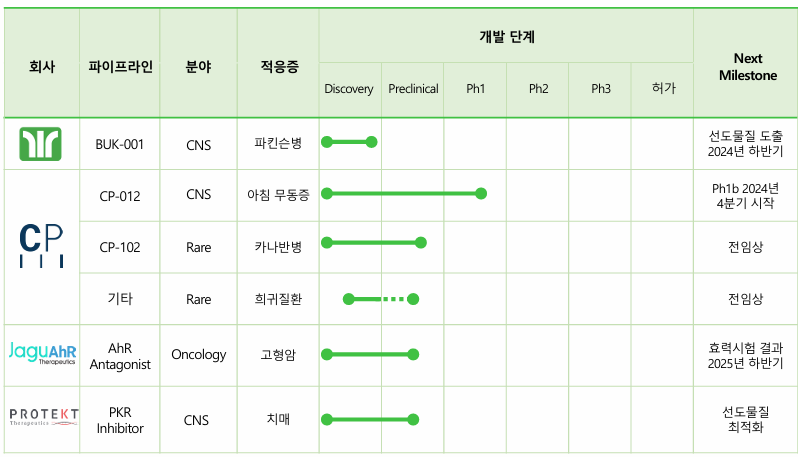
Conversely, Bukwang Pharmaceutical is advancing new drug development efforts through its headquarters and subsidiaries. "Although our headquarters' research capabilities have traditionally centered on formulations, we have reorganized the structure and initiated the development of an innovative new drug candidate, BUK-001, a 'USP30 protein-targeting therapy for Parkinson's disease,’" Kim said. "We already have a lead compound and are currently in the lead optimization stage. Once more concrete data become available, we plan to explore technology transfer or partnership opportunities. Through our subsidiary Contera Pharma, we are developing ‘CP-012,’ a candidate for treating morning akinesia in Parkinson’s disease patients, as well as a ribonucleic acid (RNA)-based treatment for rare neurological disorders,” he added.