Expanding new treatment options for patients unresponsive to PD-1/PD-L1 immunotherapy or chemotherapy

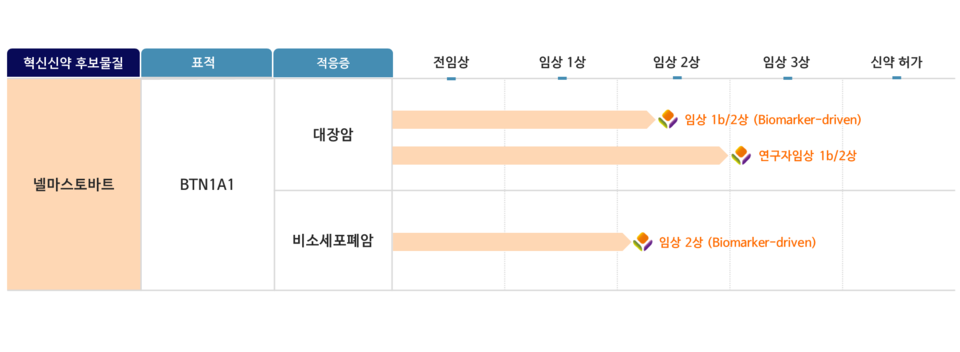
[by Kang, In Hyo] STCube has commenced a full-scale entry into the non-small cell lung (NSCLC) treatment market with its in-house developed anti-BTN1A1 immune checkpoint inhibitor candidate, ‘Nelmastobart.’ NSCLC, together with colorectal cancer, is recognized as a leading cancer type characterized by elevated BTN1A1 expression, underscoring a therapeutic area where unmet needs for immunotherapy remain substantial.
STCube announced on August 25 the submission of a Phase 2 investigational new drug (IND) application to the Ministry of Food and Drug Safety (MFDS) to assess the safety and efficacy of its combination therapy of ‘Nelmastobart and docetaxel’ in patients with advanced or metastatic NSCLC. Concurrently, the company disclosed the voluntary withdrawal of its previously approved Phase 1b/2 clinical trial targeting relapsed or refractory extensive-stage small cell lung cancer, which had received approval from the U.S. Food and Drug Administration (FDA) and the MFDS. STCube stated that this strategic decision aims to strengthen the feasibility of biomarker-driven clinical designs while concentrating development efforts on NSCLC and colorectal cancer indications, areas considered pivotal for validating BTN1A1 as a novel immuno-oncology target.
The NSCLC clinical trial will enroll patients exhibiting BTN1A1 expression (Tumor Proportion Score (TPS) ≥50%) who have previously undergone targeted chemotherapy, platinum-based chemotherapy, or immunotherapy as first-line treatment but now require second-line or subsequent therapeutic options. The study plans to recruit a total of 62 participants across seven medical institutions in Korea, including Samsung Medical Center.
The primary efficacy endpoint of the trial is progression-free survival (PFS), with secondary endpoints encompassing overall survival (OS), objective response rate (ORR), disease control rate (DCR), and duration of response (DoR). In addition, exploratory analyses will be conducted to evaluate the drug’s efficacy in relation to patient-specific factors, including BTN1A1 expression levels and the presence of genetic mutations.
Non-small cell lung cancer (안전한 바카라사이트) represents approximately 85% of all lung cancer cases. Treatment strategies vary according to the presence of genetic mutations such as EGFR, ALK, ROS1, and BRAF, for which targeted therapies are commonly recommended. In patients lacking these key genetic mutations, the current standard of care involves the use of immune checkpoint inhibitors, either as monotherapy or in combination with cytotoxic chemotherapy, guided by PD-L1 expression levels. Overall, immunotherapy constitutes roughly 60% of treatment, while targeted therapy and chemotherapy account for 30% and 10%, respectively.
In particular, relapse rates following primary treatment remain high, and therapeutic options become severely limited once resistance develops. In Korea, cytotoxic chemotherapy monotherapy, most commonly docetaxel, remains the predominant option in such cases. However, 안전한 바카라사이트 outcomes are poor, with progression-free survival (PFS) averaging only three months and overall survival (OS) approximately six months, underscoring a substantial unmet need. Against this backdrop, the company emphasizes the urgent necessity of developing new therapeutics capable of improving outcomes for patients with advanced and metastatic NSCLC who have failed existing standard treatments.
STCube reports that BTN1A1 expression is observed in 84% of squamous cell carcinoma cases and 45% of adenocarcinoma cases among NSCLC patients with a Tumor Proportion Score (TPS) of 50% or higher. The company highlights that such expression patterns render these cancers particularly suitable for biomarker-driven 안전한 바카라사이트 trials, facilitating patient selection and the verification of therapeutic efficacy.
"This clinical trial represents a strategic approach to identifying and treating high-risk patients who have failed existing standard treatments, based on the clear biomarker criterion of BTN1A1 expression. From patient selection to the development of the combination therapy regimen, the study design has been structured with global clinical applicability and technology transfer potential in mind from the outset," said Jung Hyun-jin, CEO of STCube.
"Our biomarker-driven clinical strategy is among the most critical factors assessed by global pharmaceutical companies during technology transfer discussions, and it will act as a catalyst for significantly strengthening partnership opportunities and negotiating power. In making this decision, we carefully evaluated Nelmastobart's market potential, clinical efficacy, and the broader competitive landscape, ultimately determining to shift its indication to NSCLC," Jung further commented.
STCube intends to bolster its global competitiveness by positioning BTN1A1 not only as a therapeutic target but also as a key biomarker. The company has identified colorectal cancer and NSCLC as the primary indications for development, with the objective of validating the effectiveness of its precision medicine strategy through biomarker-based 안전한 바카라사이트 trials.
"Amid the recent rise in discontinued clinical trials targeting novel immuno-oncology targets, biomarkers such as BTN1A1, which enable the selection of patient populations based on expression levels, remain exceptionally rare," Jung emphasized. "This clinical trial represents an opportunity to advance the development of biomarker-based immuno-oncology treatments, a field that has long faced bottlenecks due to challenges in clinical validation," he added.