[Interview] Stephen S. Yoo, Chief Scientific Officer (CSO) of STCube and CEO of STCube Pharmaceuticals (U.S.)
[by Kang, In Hyo] Nelmastobart, a next-generation immuno-oncology drug and anti-BTN1A1 immune checkpoint inhibitor under development, represents a genuine first-in-class novel drug candidate. By targeting ‘BTN1A1,’ a unique biomarker differentiated from ‘PD-L1,’ which is currently used to predict immuno-oncology responses, Nelmastobart holds the potential to address the significant unmet clinical needs of cancer patients who do not benefit from currently available immune checkpoint inhibitors.
In a recent interview with <THE BIO >, Stephen S. Yoo, the Chief Scientific Officer (CSO) of STCube and CEO of STCube Pharmaceuticals (U.S.), provided an overview on Nelmastobart, the company’s lead pipeline candidate currently under development. Yoo visited Korea this month in preparation for the full-scale clinical trial of Nelmastobart in patients with colorectal cancer.
‘Immune checkpoint inhibitors’ are a class of immunotherapeutic agents designed to inhibit ‘immune checkpoint proteins’, molecules that modulate or attenuate immune responses in cancer cells or immune cells. These proteins, including well-known examples such as CTLA-4, PD-1, LAG-3, and TIGIT, are expressed on immune cells and function as regulatory ‘brakes’ that suppress immune activity.
The concept of ‘immunity’ in the context of immunotherapeutic drugs extends beyond simple immune defense against invasion by non-self extrageneous matters and specifically refers to an anticancer mechanism that eliminates malignant cells through the body’s immune system, primarily using the action of ‘CD8 T cells’. Typically, cancer cells evade immune surveillance by expressing immune checkpoint proteins such as PD-L1, which transmit ‘immunosuppressive’ signals that inhibit T cell activity, neutralizing the immune system’s anticancer action.
Immune checkpoint inhibitors function by inhibiting the immunosuppressive signals, thereby reactivating CD8 T cells to recognize and target malignant cells. In this respect, immunotherapy represents a fundamentally distinct therapeutic approach compared to traditional cytotoxic anticancer drugs, as it seeks to ‘reactivate’ the body’s own immune system to attack cancer cells.
PD-L1 and BTN1A1, as referenced by Yoo, are 먹튀없는 바카라사이트 checkpoint proteins expressed in the tumor microenvironment (TME). PD-L1 is found in cancer cells and also in a range of 먹튀없는 바카라사이트 cells, where it interacts with its ligand PD-1 on CD8 T cells to induce immunosuppression. In contrast, BTN1A1 is predominantly expressed on cancer cells and binds to CD8 T cells to suppress their activation.
In essence, PD-L1 or BTN1A1 binding to CD8 T cells impairs the function of these T cells to induce 먹튀없는 바카라사이트 suppression. Accordingly, the fundamental mechanism of Nelmastobart as an 먹튀없는 바카라사이트 checkpoint inhibitor lies in the development of antibodies that block the interaction between BTN1A1 and CD8 T cells, just as the currently marketed PD-1/PD-L1 antibodies function by blocking the interaction between PD-L1 and the PD-1.
Representative PD-L1-targeting therapies include ‘Tecentriq ’ (Roche, Switzerland), ‘Bavencio ’ (Merck, Germany), and ‘Imfinzi ’ (AstraZeneca). Nelmastobart, an investigational antibody currently under development by STCube, represents a next-generation immune checkpoint inhibitor. It exerts its mechanism of action by binding to BTN1A1, thereby blocking its interaction with T cells and mitigating immune suppression.
Through preclinical studies and clinical trials on humans, we have demonstrated that BTN1A1 expression is closely associated with the suppression of T cell immune activity, and have identified its mechanism of action as a viable target for immune anticancer drugs, explained Yoo. BTN1A1 is not or minimally expressed in normal cells, but it is strongly expressed in cancer cells, making it an optimal candidate for target-driven therapeutics with excellent safety profile.
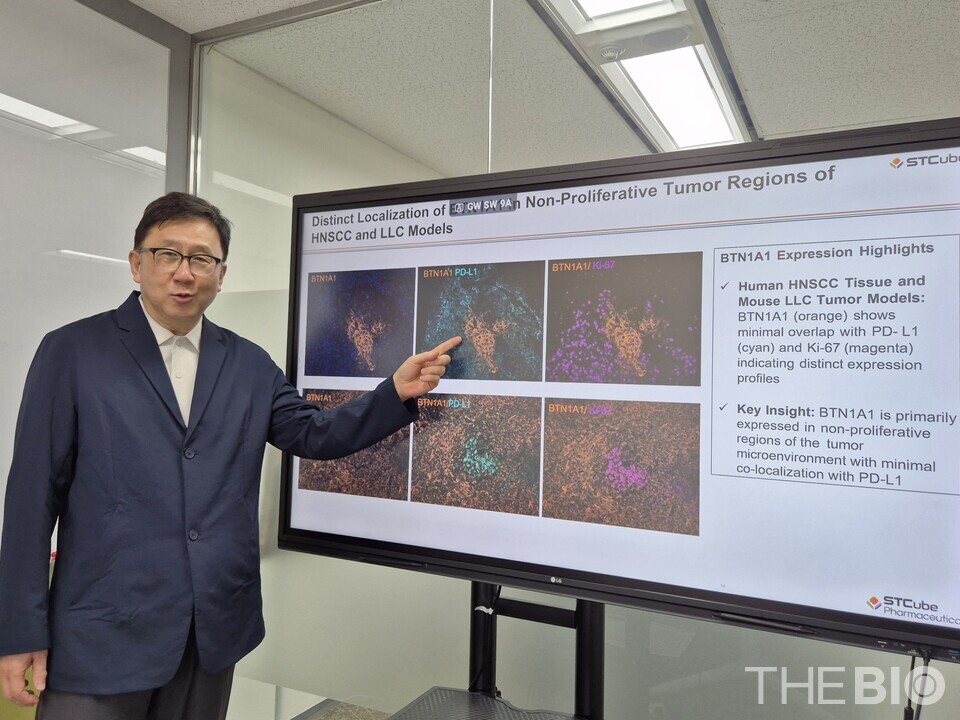
BTN1A1 exhibits a ‘mutually exclusive’ expression pattern with PD-L1, Yoo further added. Whereas PD-L1 is typically expressed in rapidly proliferating cancer cells, BTN1A1 is predominantly expressed in dormant or slowly dividing tumor cells. As such, it is gaining attention as a promising next-generation treatment target for metastatic or treatment-resistant cancers.
According to Yoo, Opal multiplex staining result revealed that even within the same tissue, BTN1A1 and PD-L1 exhibit a ‘mutually exclusive expression’ pattern, wherein the two proteins are expressed in different locations within the tissue. This pattern implies that PD-L1 is nearly absent in cells where BTN1A1 is present, and vice versa. Based on these findings, Yoo explained that BTN1A1 inhibitors may offer therapeutic benefits even in cancers that do not usually respond to PD-L1 inhibitors.
These findings suggest that BTN1A1 may operate through an 먹튀없는 바카라사이트 suppression pathway that complements that of PD-L1. Yoo thus emphasized that immunotherapies targeting BTN1A1 also hold potential in a combination therapy settings in order to address the limitations of current PD-L1-based treatments.
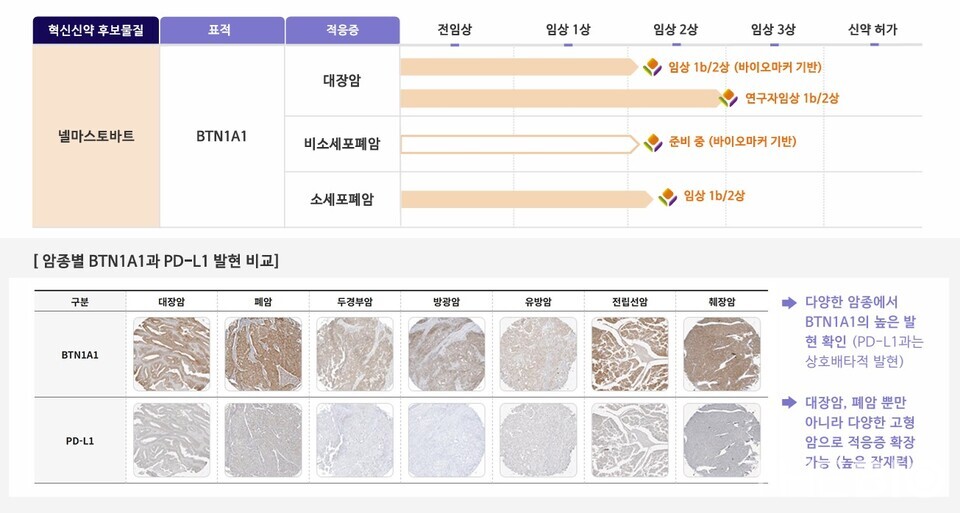
High BTN1A1 expression is observed in a number of solid tumors with low PD-L1 expression, suggesting that therapeutic benefits of BTN1A1 inhibitors can be expected in patient groups unresponsive to PD-L1 inhibitors, Yoo highlighted. Beyond the current indications under development, we have identified the potential for broader applications across additional cancer types. Clinical trials have consistently demonstrated that Nelmastobart shows a high response rate in patient groups with high BTN1A1 expression.
STCube has completed Phase 1 clinical trials evaluating ‘Nelmastobart as a monotherapy.’ Initiated in April 2022, the trial concluded with the issuance of a Clinical Study Report (CSR) in September 2024. This global clinical trial was conducted in the United States and Korea, and enrolled patients with major solid tumors like colorectal and lung cancers.
The clinical trial results indicated that 먹튀없는 바카라사이트 monotherapy did not elicit any dose-limiting toxicities (DLTs) across all administered dose levels, and no dose- or time-dependent adverse reactions were observed. According to Yoo, the treatment demonstrated an exceptional safety profile compared to other immunotherapies, with only one case (2.1%) of clinically significant treatment-related adverse events of Grade 3 or higher. The majority of adverse events were classified as mild Grade 1.
Yoo further noted that the clinical trial yielded several key research outcomes, including the clear identification of BTN1A1’s mechanism of action as a target for immunotherapy. The study also confirmed BTN1A1’s mutually exclusive and differentiated mechanism from PD-L1, demonstrated its potential for excellent treatment response, and established its viability as a biomarker.
While PD-L1 expression levels are often used as a biomarker to predict clinical responses to immunotherapy, the correlation between PD-L1 expression and therapeutic efficacy has not always been consistent in all clinical trials, Yoo said. A high level of PD-L1 expression does not necessarily translate into a better response to immunotherapy, and it was observed that some patients with low PD-L1 expression still experience treatment benefits, indicating that PD-L1 alone may be an inadequate biomarker.
At present, there is no dependable biomarker capable of predicting and determining the level of patient response to immunotherapy in advance, he further emphasized. STCube seeks to develop the first genuine biomarker-driven novel drug in immunotherapy by targeting BTN1A1 with Nelmastobart.

Yoo strives to complete the development of a blockbuster novel therapeutic by targeting high-incident cancers such as colorectal and lung cancers through a ‘biomarker-driven’ strategy. This approach, rooted in the principles of precision medicine, selects patients based on the expression of specific biomarkers such as BTN1A1, rather than non-selectively administering drugs to any and all patients, and develops or administers treatments tailored to that selected group.
As an immune-suppressive agent that exerts its function independently of PD-L1 expression, Nelmastobart is garnering attention as a promising candidate in the development of next-generation immune checkpoint inhibitors. Currently, STCube is conducting three clinical trials involving combination therapies using Nelmastobart. These include a phase 1b/2 clinical trial of the Nelmastobart and ‘capecitabine’ combination, primarily for colorectal cancer; a domestic phase 1b/2 clinical trial evaluating a triple combination of Nelmastobart, ‘TAS-102’ and ‘bevacizumab’; and a global Phase 1b/2 clinical trial testing Nelmastobart in combination with ‘paclitaxel’ for small cell lung cancer.
The phase 1b/2 clinical trial of the Nelmastobart and ‘capecitabine’ combination for colorectal cancer is being conducted as a third-line treatment for metastatic colorectal cancer in patients who have failed or are unable to receive standard chemotherapy (oxaliplatin and irinotecan) at least twice. All 51 patients have been enrolled and treated and are currently undergoing follow-up observation. In the phase 1b clinical trial (12 patients), two patients with microsatellite stable (MSS) colorectal cancer achieved partial response (PR), while others showed stable disease (SD), resulting in an objective response rate (ORR) of 17% and a clinical benefit rate (CBR) of 66.7% at 16 weeks (4 months).
The domestic phase 1b/2 clinical trial of Nelmastobart, ‘TAS-102’, and ‘bevacizumab’ triple combination for colorectal cancer is scheduled to begin administration within the first half of the year. The trial targets patients with metastatic or recurrent colorectal cancer that is refractory or intolerant to chemotherapy based on ‘oxaliplatin’ and/or ‘irinotecan’.
The first patient in the phase 1b/2 clinical trial evaluating the triple combination for colorectal cancer is scheduled to begin treatment in June, Yoo said. As the phase 1b clinical trial is designed to assess the safety of the triple combination with Nelmastobart, we anticipate a prompt clearance of dose-limiting toxicity (DLT), allowing us to proceed to phase 2 in August and initiate treatment in patients with BTN1A1-positive expression.
The phase 2 clinical trial for colorectal cancer is designed to include a biomarker-driven patient selection study, and we therefore anticipate improved response outcomes compared to the previous investigator-initiated trial, he further commented. We are currently preparing an investigational new drug (IND) application to begin a clinical trial in non-small cell lung cancer during the second half of the year, and the results of the phase 1b/2 trial for colorectal cancer, including phase 2 data, will be presented at the Society for Immuno-Oncology of Cancer (SITC) in November.
STCube has secured adequate funding required for planned clinical studies, thereby minimizing the burden of funding future clinical trials. Earlier this year, the company successfully resolved its issue of ‘loss before corporate tax,’ enabling it to avoid being listed on the ‘administrative watch list’. Notably, STCube raised a total of KRW 81.4 billion (approximately USD 58.8 million) through a paid-in capital increase in 2024, reinforcing its efforts to secure funding for the clinical trials and enhance the overall financial stability.
STCube’s Chief Financial Officer (CFO), Park Jun-yong, who also participated in the interview, stated that [t]here are currently no plans for additional fundraising, and noted, As of the end of the Q1 2025, the company holds around KRW 85 billion in cash and cash equivalents, a level sufficient to support the planned phase 2 clinical trials with financial stability. He added, We have developed a comprehensive financial strategy that accounts for various clinical cost scenarios. At this stage, we expect the clinical trial to proceed smoothly as scheduled.
STCube identified the clinical data of 먹튀없는 바카라사이트 as a key element in ongoing technology transfer (L/O) negotiations. The company emphasized that robust clinical data accumulated from the progression of full-scale clinical trials in colorectal cancer will provide the company with concrete opportunity to pursue a licensing-out strategy.
We are currently receiving meeting requests from various global companies, and we plan to attend ‘BIO USA’ this year to conduct key business meetings and actively engage in oncology conferences, Yoo expressed. In particular, we are in relatively advanced discussions with certain companies, and we intend to move into more formal negotiations as the phase 1b/2 clinical trials for colorectal cancer progress.
Technology transfer is important not only in terms of its monetary scale, but also in identifying the right partner, and thus, it is essential to define our business strategy accordingly, Yoo further emphasized. STCube is actively seeking partners capable of rapidly commercializing the drug and fostering the long-term growth of Nelmastobart, and we indend to focus on accelerating clinical trials to achieve timely outcomes.

