- Professor Park Kyong-hwa of Korea University Anam Hospital joins a media session hosted by 온라인바카라 Lilly Korea
- Stresses importance of ‘Verzenio combination’ adjuvant therapy for high-risk early-stage 온라인바카라 patients
- Rising financial burden from relapse in high-risk groups… “Cost-effectiveness must be considered”
- 온라인바카라 Lilly: “Applying for additional benefits by weighing clinical efficacy, drug pricing, and more”

[by Yu, Suin] “It would cost about KRW 50 million (approximately USD 35.9 thousand) to continue treatment for around two years with Verzenio (abemaciclib) + endocrine therapy combination therapy. Considering that the annual treatment cost for patients with metastatic breast cancer exceeds KRW 50 million, wouldn’t it be socially more beneficial to focus efforts on actively treating high-risk patients with early-stage breast cancer?”
Professor Park Kyong-hwa of the Department of Oncology at Korea University Anam Hospital made this statement during a media session hosted by Eli Lilly Korea on the morning of July 16 at the HJ Business Center Gwanghwamun branch in Seoul. She emphasized the need to expand insurance coverage for ‘Verzenio.’ Although Eli Lilly Korea has twice submitted applications to extend coverage for Verzenio to include early-stage breast cancer as an indication, the proposals were rejected on the grounds that coverage criteria had not yet been established.
온라인바카라 cancer exhibits varying prevalence and survival rates depending on whether the hormone receptors (HR) and human epidermal growth factor receptor 2 (HER2) are negative or positive. Among them, patients with HR+2 and HER2- represent the largest proportion, accounting for approximately 73% of all 온라인바카라 cancer cases.
Verzenio is a CDK 4/6 inhibitor approved for the treatment of ‘early-stage’ and ‘metastatic breast cancer.’ It was initially approved in Korea in May 2019 for the treatment of ‘HR+/HER2- advanced or metastatic breast cancer.’ In 2022, its indications were expanded to include adjuvant treatment, in combination with endocrine therapy, for adult patients with ‘HR+/HER2- lymph node-positive early breast cancer at high risk of recurrence.’ Verzenio is the first CDK 4/6 inhibitor to receive approval for use in ‘high-risk early breast cancer with recurrence potential.’
온라인바카라 cancer is the leading cause of cancer-related mortality among women worldwide. In Korea, the incidence of 온라인바카라 cancer has risen by approximately 1.6 times since 1990. Furthermore, the number of patients diagnosed with early-stage 온라인바카라 cancer, including stage 3 patients classified as high-risk, has more than doubled over the past 20 years.
Breast cancer is often referred to as a ‘good cancer’ due to its cure rate, exceeding 95%, when detected at an early stage. However, once recurrence occurs, the likelihood of achieving a cure significantly diminishes, underscoring the need for ongoing support for cancer survivors. A major concern is that 2 out of 10 patients experience relapse, with many cases of recurrence and metastasis emerging as late as 10 years after patients have been considered cured. These events are more common among relatively young patients, and the recurrence rate is notably higher in the high-risk subgroup compared to the overall population of early breast cancer patients.
The high-risk group of patients with HR+/HER2- 온라인바카라 cancer exhibits the highest rate of recurrence in the first one to two years. Moreover, early-stage 온라인바카라 cancer may involve micrometastases that persist even after surgical intervention, highlighting the critical need for ongoing and proactive treatment.
“The majority of breast cancer patients in Korea are in their 40s and 50s. Due to their relatively young age, many face challenges in returning to work, managing household responsibilities, and raising children. They often experience emotional distress, fearing recurrence and uncertainty about the future,” Park emphasized. “This situation results in reduced income and productivity, leading to broader societal and economic losses. If we are to assess ‘cost effectiveness’ from a long-term and social perspective, I believe these factors must be given greater consideration.”
It is reported that up to 30% of high-risk patients with early-stage 온라인바카라 cancer experience recurrence. In particular, studies indicate that among patients with the HR+/HER2- subtype who are classified as high-risk based on factors like lymph node metastasis or genetic testing, the recurrence rate within 10 years ranges from 20% to 30%. Industry experts suggest that actively intervening in these high-risk cases at an early stage is more cost-effective from a healthcare financing perspective than adopting a sequential treatment approach after metastasis has occurred.
“Proactively investing in high-risk early-stage patients may be more beneficial than adding medications sequentially after metastasis has occurred. For HR+/HER2- patients, once metastasis develops, treatment typically involves sequential administration of drugs, but the current median survival period is approximately five years,” she further commented. “If we assume that 30% of high-risk patients experience recurrence, the health insurance system would need to sustain financial support for at least five years.”
“The clinical utility of Verzenio has been validated through several studies, and it is receiving strong interest and expectations from medical professionals and patients in real-world clinical settings,” she added. “Administering Verzenio in combination with endocrine therapy for two years significantly reduces the risk of recurrence and metastasis compared to endocrine therapy alone. Moreover, clinical trials indicate that the difference in treatment outcomes continues to widen over the long term.”
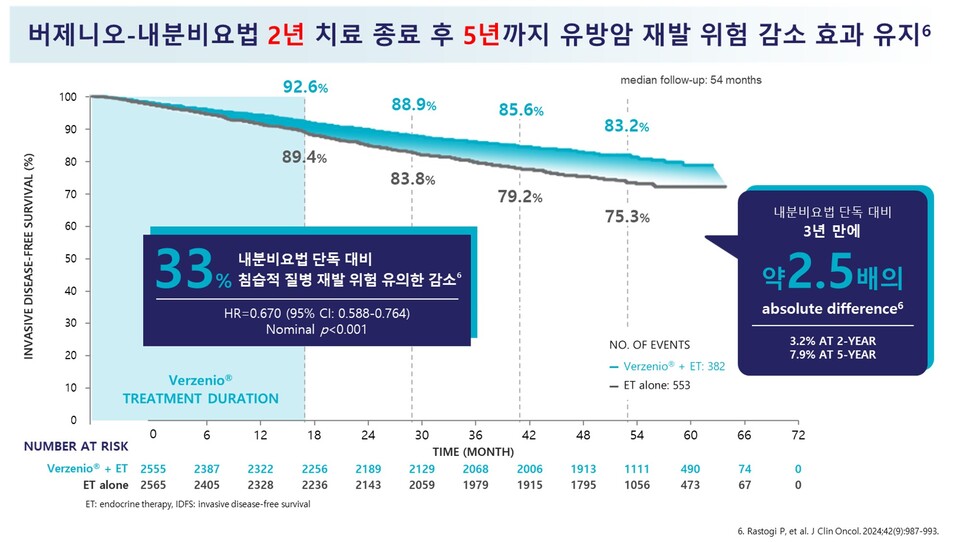
The effectiveness of ‘Verzenio combination therapy’ has also been confirmed by long-term clinical data. In the five-year follow-up results of the ‘monarchE’ Cohort 1 clinical study, which targets patients with early-stage breast cancer at high risk of recurrence (as defined by clinical and pathological criteria), the Verzenio combination therapy reduced the risk of recurrence and death (invasive disease-free survival, IDFS) by approximately 33% compared to endocrine therapy alone (HR: 0.670, 95% CI: 0.588-0.764; p<.001).
The risk of distant recurrence and death (distant recurrence-free survival, DRFS) was also reduced by approximately 33% with 온라인바카라 combination therapy compared to endocrine therapy alone (HR: 0.665, 95% CI: 0.577-0.765; p<.001). Notably, the difference from the placebo widened over time, with the risk reduction rate increasing as the follow-up period progressed.
In the analysis of the overall patient population, the 온라인바카라 + endocrine therapy combination consistently demonstrated favorable results. The reduction in the risk of recurrence and death compared to endocrine therapy alone gradually increased in the second, third, fourth, and fifth years. Additionally, the absolute differences in IDFS and DRFS continued to widen throughout the five-year follow-up observation point.
“To my knowledge, health insurance coverage for Verzenio is applied in most foreign countries,” Park stated. “I sincerely hope that coverage for Verzenio as adjuvant therapy will be promptly expanded in Korea so that high-risk early-stage breast cancer patients can also benefit from insurance support. This would ensure equal access to treatment for all patients, regardless of their income level.”
“Leading clinical guidelines, including those from the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology (ASCO), and the Korean Breast Cancer Society, recommend a two-year course of Verzenio + endocrino combination therapy as postoperative adjuvant treatment for patients with HR+/HER2- high-risk early-stage breast cancer,” said Kwon Mi-ra, Head of Oncology Business Unit at Eli Lilly Korea. “We have submitted supporting clinical data to the Health Insurance Review & Assessment Service (HIRA) based on this evidence.”
“Lilly is taking into account not only the clinical efficacy of the treatment but also drug pricing in the government’s review process,” Kwon further noted. “We are also planning to release seven-year long-term follow-up data and overall survival (OS) outcomes in the second half of the year.”
