- [Interview] Lee Min-woo, Founder and CEO of 해시 게임 바카라
- Pursuing global leadership in the 해시 게임 바카라 platelet market through mass production and IPO plans
- Targeting to surpass Japan's Megakaryon with concurrent clinical trials and IPO by 2028

[by Kang, In Hyo] In January 2022, the American Red Cross announced its first-ever ‘national blood crisis,’ highlighting the urgent need to secure a stable blood supply. Sparked by the spread of the COVID-19 Omicron variant, the situation represented the most severe blood shortage in years.
Blood, a vital biological component of the human body, consists of four main elements: plasma, red blood cells, white blood cells, and platelets. Among these, only red blood cells and platelets can be mass-produced using scientific technology. Platelets, in particular, can only be obtained through blood donation, yet their supply has been unable to meet demand due to a decline in donors caused by an aging population and the spread of infectious diseases like COVID-19. As a result, ‘artificial blood’ is being developed by synthesizing specific blood components rather than replicating whole blood.
Established in October 2021 at the height of the COVID-19 pandemic, DewCell is the only company in Korea specializing in the development of artificial platelets. The name DewCell combines ‘dew,’ symbolizing the smallest unit of water, with ‘cell,’ the smallest unit of life. It reflects the belief that, just as dew collects to form rivers and oceans and cells unite to create a single living organism, the small ideas and efforts of its employees can bring about meaningful changes in the lives of patients and their families.
DewCell, a developer of artificial platelets produced through stem cell differentiation, aspires to grow into a global company offering products accessible to anyone, anywhere, at any time. This vision is encapsulated in its slogan, "For Everyone Who Needs It."
<THE BIO> recently sat down with 해시 게임 바카라 founder and CEO Lee Min-woo to discuss the company's growth trajectory and future vision.
◇Stem cell-derived 해시 게임 바카라 platelets overcome blood type and ethnic barriers
One of DewCell’s core business areas is the development of artificial platelets, similar to those derived from human blood, through stem cell technologies. The company's fundamental vision is to produce blood derived and differentiated from stem cells with a quality equivalent to that of the human body's own blood.
"When we founded the company, the COVID-19 pandemic had caused a severe blood shortage. Meanwhile, the cell therapy sector was experiencing rapid growth, driving major advances in stem cell differentiation and culture technologies. We believed that producing artificial blood from stem cells would be highly competitive and could establish a new paradigm," Lee remarked. "Among the various types of artificial blood under development, such as red blood cells and platelets, platelets offer greater potential for pharmaceutical business scalability (transfusion, regenerative medicine, advanced biometrials, etc), which is why we prioritized developing stem cell-derived artificial platelets."
Red blood cells carry surface antigens, making compatibility between blood types A, B, and O essential. Although platelets also contain antigens, Lee noted that uniformly manufactured artificial platelets derived from stem cells can greatly minimize restrictions related to blood type or ethnicity. "DewCell's goal is to produce platelets of consistent quality that can be administered safely and reliably, regardless of a patient’s age, race, or blood type," he emphasized.
DewCell utilizes human induced pluripotent stem cells (hiPSCs) in its development process. While the research initially began with cells extracted from humans, the company now produces artificial platelets using standardized ‘production cell lines.’ Unlike autologous cell therapies, which require the collection of cells from each individual patient, this approach enables master/working cell bank (MCB, WCB) mass production from a production cell line, ensuring high quality and a stable supply.
Although DewCell is the sole developer of artificial platelets in Korea, it faces competition internationally. Key examples include Japan’s Megakaryon and the United States’ Stellular Bio.
"Japan's Megakaryon is the world's leading company in this field, having already entered Phase 1 clinical trials for artificial platelets in 2019, which are still ongoing," Lee commented. "In the United States, a large-scale artificial blood derived from stem cells project is also underway, led by the Department of Defense."
"Platelet Bio, which originated from a U.S. Department of Defense project, later rebranded its name as Stellular Bio. The company is currently producing artificial platelets at the preclinical stage and focus on regenerative medicine for ocular surface disease(OCD)," Lee further explained.
Several companies in Korea, such as Artblood, YiPSCELL, and RedGene, are engaged in the development of artificial red blood cells. "As the sole developer of artificial platelets in Korea, DewCell intends to launch its products in Korea at the earliest opportunity to prove their viability in real-world patient treatment. Once we confirm that our artificial platelets match the efficacy of blood-derived platelets, we expect to attract international interest and collaboration requests," he further added.
“While most leading artificial platelet development companies in Japan, the United States, and Europe were founded by people from academia, our company was established by someone from the industrial sector,” Lee emphasized. “Our advantage lies in the ability to transition swiftly from research to actual commercialization and establishment of a production system.”
◇Developing three pipelines based on ‘en-aPLT’-based artificial platelet pipelines to target Korean and international markets
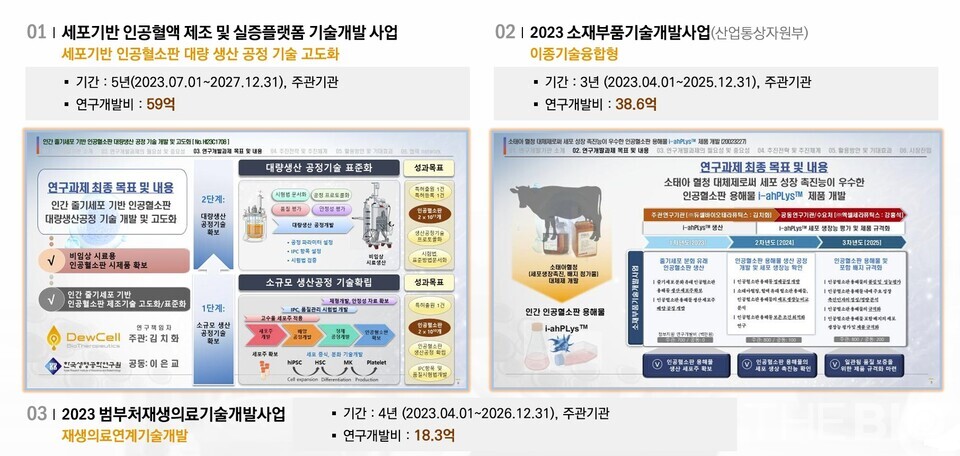
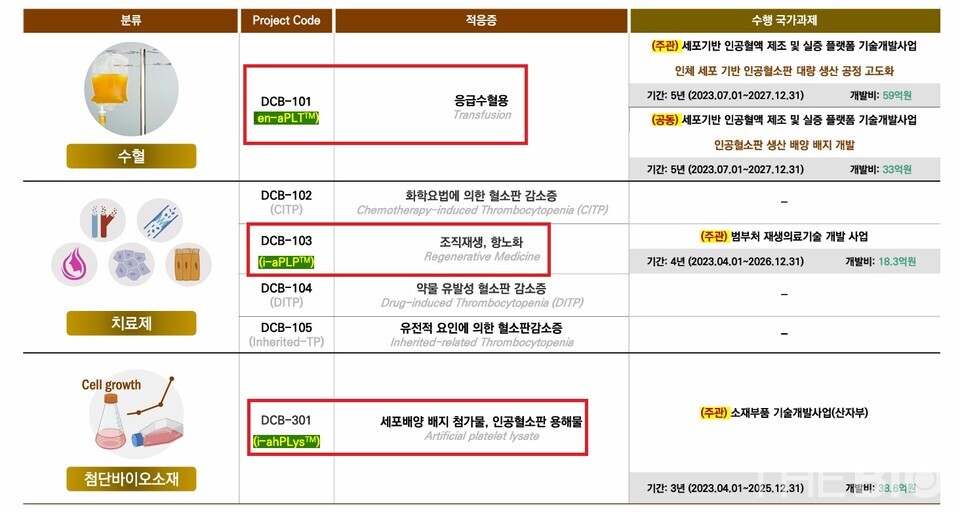
DewCell has developed a proprietary artificial platelet production platform, 'en-aPLT,' and is advancing three pipelines utilizing this platform: 'Transfusion (development code DCB-101),' 'Therapeutic use for regeneration (DCB-103),' and 'Advanced Biomaterial (DCB-301).'
Platelets play a crucial role in primary hemostasis, rapidly aggregating to stop bleeding when vascular injury occurs. At the same time, they release a variety of regenerative factors essential for tissue repair, contributing to processes such as cell proliferation, angiogenesis, and the regulation of inflammatory responses. Notably, platelet granules contain key growth factors, such as PDGF, VEGF, EGF, and TGF-β, that are vital for cell growth and tissue regeneration, thereby facilitating the healing and regeneration of damaged tissues.
"In Korea, for transfusions, all blood is obtained through donations, with platelet supplies managed by the Korean Red Cross. Due to the substantial challenges in securing adequate platelet supplies, our initial project focuses on manufacturing and providing artificial platelets for patients requiring transfusions," Lee remarked.
"Our second focus is the development of therapeutics. Artificial platelets carry a range of tissue regeneration factors. When platelets accumulate at damaged sites, they form scabs, and the subsequent formation of new skin is driven by the regenerative capabilities of these platelets," he further explained.
"Leveraging this tissue regeneration capability, as a representativ e project in the filed of advanced regenrative medicine, we are developing treatments by prioritizing osteoarthritis(OA) as an aging-related disease that requires tissue regeneration. In addition, we are conducting basic research to extend its application to ophthalmology, dermatology, plastic surgery, and dentistry," he added.
Lastly, we are working on the development of 'cell culture media supplement.' As the cell therapy market expands, the demand for cell culture media is also increasing. While fetal bovine serum (FBS) was traditionally the primary choice, there is now a shift toward the use of ‘chemically defined media(CDM),’ chemically composed of precisely characterized chemical components.
"Given the specific requirement of cell therapies administered to humans, DewCell is developing artificial platelet lyaste contain various cell growth promotion factors, as a replacement for FBS and hPL(human blood platelet-derived)" Lee stated.
Platelets for transfusions are particularly attracting considerable national attention. "In July 2023, the 'Korea Cell-Based Artificial Blood Project (KCABP)' was established under the leadership of the Ministry of Health and Welfare, with joint funding from five government bodies: the Ministry of Trade, Industry and Energy, the Ministry of Science and ICT, the Ministry of Food and Drug Safety, and the Korea Disease Control and Prevention Agency," he explained.
"The project is advancing a government initiative to develop artificial red blood cells and artificial platelets. Within this framework, DewCell was exclusively selected for the 'Development and Advancement of Mass Production Process Technology for Human Stem Cell-Based Artificial Platelets' project, securing around KRW 6 billion (approximately USD 4.3 million) in research and development funding over a five-year period," he further commented.
"Advanced nations such as Japan, the U.S., and those in Europe have already identified this technology as a 'national strategic asset,' investing heavily and pursuing commercialization from the early stages," Lee emphasized. "In contrast, Korea is somewhat behind in this field, making national attention and support indispensable."
해시 게임 바카라 platelets are classified as cutting-edge biopharmaceuticals, necessitating clinical trials for both transfusion and therapeutic applications. Conversely, cell culture media supplement fall under the category of advanced biomaterials and are therefore exempt from clinical trial requirements.
"For transfusion use, artificial platelets have well-defined clinical evaluation standards. Similar to how platelet counts are measured in routine health checkups, platelet function tests can also be performed when necessary. With clinically standardized analytical methods already established, our primary task is simply to verify that the artificial platelets perform their intended functions in the human body," Lee explained.
"Since conducting clinical trials on patients with severe bleeding is challenging, current studies (DCB101) are being carried out on patients with thrombocytopenia. Functional equivalence can be demonstrated simply by assessing whether platelet counts return to normal levels following transfusion in patients with low platelet counts," he further added.
Therapeutic artificial platelets (DCB-103) follow the same development process as conventional new drugs. "Phase 1 clinical trials involve patients to evaluate toxicity, safety, and potential side effects. Phase 2 focuses on assessing efficacy, while Phase 3 is conducted on a larger patient population to verify effectiveness prior to commercialization," Lee explained.
"Although clinical trials for osteoarthritis treatments are feasible, we have not yet finalized specific plans," he continued. "In animal studies, we administered the treatment to osteoarthritis-induced models, confirming pain-relief anti-inflammatory effects and its ability to regenerate damaged tissues. Based on these findings, we assessed its therapeutic efficacy and intend to apply this methodology in the clinical trial phase, in accordance with international standards."
We have successfully developed a prototype of the cell culture media supplement. "We have signed a letter of intent (LOI) with Germany's PL BioScience for the supply of raw materials development artificial platelet lysate," Lee expressed. "The agreement, signed on May 9, is not a formal export contract but is valued at approximately USD 2 million."
◇Targeting 50-liter mass production with KRW 20 billion Series C funding… IPO planned for 2028
해시 게임 바카라 is currently seeking KRW 20 billion (approximately USD 14.4 million) in Series C funding, coming just one year after securing a KRW 12.5 billion Series B round in July 2024. The company aims to close the Series C round by the end of September.
"While last year's funding was primarily allocated to R&D, the Series C round is intended to secure mass production facilities(cGMP grade). As our technology has advanced considerably in just one year, this round is focused on laying the foundation for sacle-up for mass production," Lee explained.
"At present, DewCell produces 26 liters of artificial platelets per month using four 2-liter bioreactor and two 5-liter bioreactor. To scale up and shift to full-scale production, the use of a 50-liter bioreactor will be essential," he added.
"Our ultimate goal for clinical and commercial product is to produce using 50-liter and 200-liter bioreactor. To this end, we will be introducing a 50-liter bioreactor in October this year, with the aim of achieving successful culture by year’s end. If we meet this goal, we will become the first company in the world to successfully cultivate 50-liter artificial platelets," he further emphasized.
According to Lee, Japan's Megakaryon operated four 10-liter incubators, producing a total of 32 liters for clinical trial, a scale that other companies have yet to match. "DewCell currently operates 2-liter and 5-liter bioreactor. By introducing a 50-liter bioreactor this year, we will set a successful precedent for a large-scale production using manufacturing facilities that exceed those of Megakaryon," he emphasized.
"Megakaryon was recently acquired by Sysmex, a Japanese clinical equipment company. Earlier this year, Sysmex outlined Megakaryon's project vision, announcing plans to temporarily halt ongoing clinical trials, develop automatic production process (closed platelet production system), and planning a submission for the 2029 clinical trial," he added.
"Megakaryon initially began developing artificial platelets but it would have been necessary to develop a mass production for many patient clinical trial. In contrast, DewCell has pursued process development from the outset with a 50-liter target in mind. If everything proceeds as planned, DewCell could enter clinical trials ahead of Megakaryon, or even take the lead," Lee further commented.
해시 게임 바카라 has set a mid- to long-term objective of acquiring two 50-liter and two 200-liter bioreactor within the next three years, reaching a monthly production capacity of 500 liters. This would allow the company to complete a full-scale mass production system capable of supplying products directly to patients.
"Once the mass production facility is in place, we intend to generate revenue primarily through sales of artificial platelet lysate produced at the site. We plan to initiate clinical trials for transfusion and therapeutic use by 2028 and pursue an initial public offering (IPO)," Lee said. "At the time of the IPO, we aim to be listed as a biotech company conducting two clinical trials, one for transfusion use and one for therapeutic use, while achieving at least KRW 6 billion in lysate sales."
Upon completion of the Series C funding round, DewCell plans to appoint an underwriter and start full-scale IPO preparations. While a materials, components, and equipment track is under consideration for the company’s related projects, Lee noted that DewCell's primary focus lies in therapeutics and transfusions, making a technology-special listing another viable option.
“DewCell’s business model is centered on directly developing and commercializing artificial platelets rather than pursuing out-licensing,” Lee further emphasized. “We will establish a stable cash flow through business avenues capable of generating revenue even prior to entering clinical trials.”

