- Exploring technology export opportunities for the second pipeline ‘바카라 토토 사이트201’ to global pharmaceutical companies
- 바카라 토토 사이트201, a novel colorectal cancer treatment, advances in development following domestic Phase 1/2 clinical trial approval in late 2025
- Building a complete data package…Establishing a solid scientific foundation for clinical trials
- Preparing to apply for technology evaluation and launch IPO process soon…Targeting a technology-specific listing on KOSDAQ within the year

[by Kang, In Hyo] 바카라 토토 사이트, which successfully secured more than KRW 60 billion (approximately USD 40.7 million) in large-scale investment last year, is once again pursuing global licensing-out opportunities this year. In parallel, the company is actively advancing preparations for a public listing with the aim of completing an initial public offering (IPO). 2026 is expected to be a critical inflection point for 바카라 토토 사이트, marking a critical phase in both its business expansion strategy and the restructuring of its financial base.
According to industry sources on January 14, NEX-I is exploring licensing-out (L/O) opportunities with global pharmaceutical companies for its second pipeline candidate, ‘NXI-201 (development code).’ Previously, NEX-I had validated the effectiveness of its proprietary immuno-oncology target discovery platform by successfully licensing out its first preclinical pipeline, ‘NXI-101 (development code),’ to Japan's Ono Pharmaceutical in 2024.
Both NXI-101 and NXI-201 target novel tumor-derived immunosuppressive proteins identified through NEX-I's core proprietary platform, ‘ONCOCINE,’ an immuno-oncology target discovery platform. Specifically, NXI-101 targets ‘ONCOCINE-1,’ while NXI-201 is designed to inhibit ‘ONCOCINE-2.’ NXI-101 is being developed as a first-in-class treatment candidate for non-small cell lung cancer, whereas NXI-201 is under development for the treatment of colorectal cancer.
Similar to ONCOCINE-1, NXI-201 targets ONCOCINE-2, a protein that facilitates cancer cell survival. Its biological significance is particularly pronounced in so-called ‘cold tumors,’ which are characteristically resistant to immunotherapy. ONCOCINE-2 functions by inhibiting the infiltration of immune cells like T cells into the tumor and creates a suppressive immune environment around it.
NXI-201, an ONCOCINE dual-target antibody, is designed to neutralize key regulatory factors in the tumor microenvironment (TME) that are major contributors to ‘refractory’ immunotherapy. By modulating these immunosuppressive components, the candidate aims to transform ‘cold tumors’ with a weakened immune response into ‘hot tumors’ with a high level of immune activity, thereby reactivating and restoring effective anti-cancer immune responses.
"NXI-201 is drawing significant interest from pharmaceutical companies seeking to develop treatments for patient populations that are refractory to immunotherapy. As with NXI-101, we are meticulously preparing a comprehensive data package designed to address clear unmet medical and market needs, with the goal of successfully proposing the drug for partnerships with major global pharmaceutical companies," emphasized Yoon Kyoung-wan, CEO of NEX-I.
바카라 토토 사이트201 has completed nonclinical toxicity studies (GLP Tox) in collaboration with its contract development and manufacturing organization (CDMO) partner, Samsung Biologics. NEX-I obtained Investigational New Drug (IND) approval for a Phase 1/2 clinical trial of 바카라 토토 사이트201 from the Ministry of Food and Drug Safety (MFDS) in late 2025.
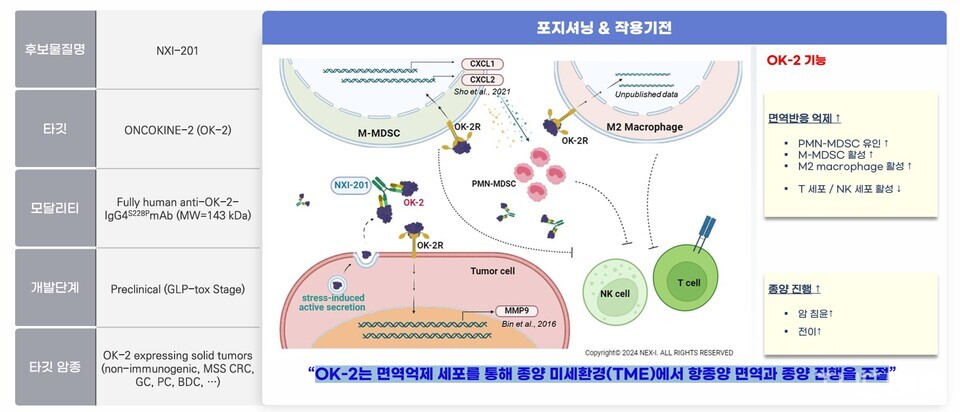
Unlike 바카라 토토 사이트101, which achieved a global licensing-out agreement at the preclinical stage, 바카라 토토 사이트201 is supported by a more comprehensive and mature data package, developed on the basis of extensive experience gained through rigorous due diligence with Ono Pharmaceutical in Japan. NEX-I explained that this strengthened the scientific foundation for clinical trials.
"With NXI-201, we are pursuing a strategy of generating revenue through technology transfer at the preclinical or early clinical stage, grounded in core mechanism data validated through the discovery process and supported by translational clinical studies. We have strategically assembled a data package that is essential for technology transfer to major global pharmaceutical companies and have continuously worked to enhance its completeness. At the same time, we are exploring customized partnerships aligned with our in-house R&D capabilities and are actively conducting business development (BD) activities to evaluate the suitability of such partnerships," a NEX-I official said.
Having successfully secured substantial investment in the previous year, NEX-I is now preparing for an IPO this year. The company plans to imminently apply for a technology assessment and formally begin preparations for the IPO process. The company previously appointed Korea Investment & Securities as the lead underwriter for its 2024 IPO and intends to enter the KOSDAQ market through a ‘technology-special listing,’ with the IPO targeted to be completed within the year.
"Our immuno-oncology pipeline for refractory cancer treatment, NXI-201, was selected last year for a new research grant from the Korea Drug Development Fund (KDDF). Through this, the company will secure research funding to advance the development of NXI-201 and will actively pursue R&D with the objective of progressing to global clinical trials and eventual commercialization," the NEX-I official remarked.
“Following the selection of NXI-101, whose technology was licensed to Ono Pharmaceutical in March 2024, as a ‘KDDF Excellent Project’ in the same year, NXI-201, a follow-up pipeline derived from the same immuno-oncology target discovery platform, has also been selected for support under the KDDF. This recognition underscores the competitiveness and development potential of our company’s therapeutic technologies targeting ‘immuno-oncology refractory factors.’ Leveraging KDDF funding, we will accelerate the R&D of NXI-201 to deliver new treatment options to patients and advance our expansion into the global market,” the official further said.

