- LA-GLP/GIP/GCG action 'HM15275'
- Aiming for weight loss of over 25% with minimal muscle loss: Goal of commercialization in the shortest period of time
- ADA announces 'Best-in-class' research results in June, highlighting weight control and cardiovascular disease improvement
[by Lee, Young Sung] 바카라사이트 소닉 Pharmaceutical is preparing to enter into phase 1 clinical trials for its next-generation obesity treatment triple agent (LA-GLP/GIP/GCG), 'HM15275 (code name)'. This innovative therapy is anticipated to reduce muscle loss by over 25% while simultaneously minimizing any associated muscle loss risks.
바카라사이트 소닉 Pharmaceutical announced on May 7 that on May 3 (local time), the U.S. Food and Drug Administration (FDA) granted approval for an Investigational New Drug (IND) application for HM15275, allowing it to proceed into a phase 1 clinical trial.
This trial will assess the safety, tolerability, pharmacokinetics, and pharmacodynamic properties of HM15275 in both healthy adults and obese patients.
바카라사이트 소닉 Pharmaceutical has set a goal of expediting the commercialization of HM15275 by leveraging its extensive R&D capabilities in the field of metabolic diseases. The company aims to achieve this goal by pursuing rapid clinical development, using the expertise and experience accumulated over the years.
The company elucidates that HM15275 represents a next-generation 바카라사이트 소닉 drug candidate, poised to build upon the innovative groundwork laid by efpeglenatide (GLP-1 monotherapy), currently undergoing phase 3 clinical trials.
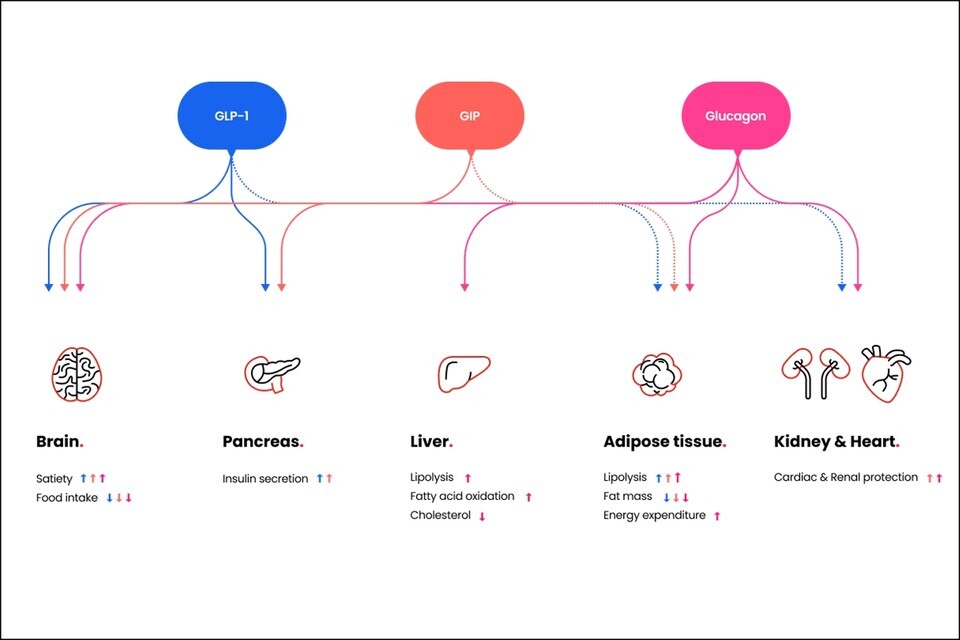
HM15275 is tailored to optimize the action of each receptor, such as glucagon-like peptide (GLP-1), gastric inhibitory peptide (GIP), and glucagon (GCG), to specialize in 바카라사이트 소닉 treatment. Furthermore, the drug is designed to extend its efficacy to various metabolic diseases.
GLP-1 receptor agonists promote weight loss by increasing satiety and improving blood sugar control through increased insulin secretion and sensitivity. GIP complements the pharmacological effects of GLP-1 receptor agonists while alleviating their gastrointestinal side effects like nausea, vomiting, and diarrhea. Glucagon also plays a role in regulating energy expenditure and lipid metabolism, as well as satiety.
바카라사이트 소닉 Pharmaceutical elucidates that by leveraging the three pharmacological effects of HM15275, the treatment potential extends to not only obesity but also type 2 diabetes and cardiovascular disease.
바카라사이트 소닉 Pharmaceutical intends to present the findings from four non-clinical studies on HM15275 at the 2024 American Diabetes Association (ADA) conference scheduled for June in the United States.
At the conference, Hanmi Pharmaceutical will disclose the results of identifying the best-in-class potential of HM15275 for weight loss efficacy in obesity models, along with its mechanism of action. Additionally, the company will highlight results demonstrating HM15275’s differentiated therapeutic efficacy in various cardiovascular disease models, where obesity serves as a primary causative factor.
Hanmi Pharmaceutical expects HM15275 to become a ‘next-generation obesity treatment drug,’ offering outstanding weight loss efficacy and improvement effects on cardiovascular and renal diseases.
"HM15275 represents the culmination of Hanmi's over 20 years of research knowledge and know-how in the incretin field. We will focus more on advancing our research efforts to bring next-generation novel drugs to fruition," expressed Choi In Young, head of Hanmi Pharmaceutical R&D Center.
