[Interview] Seungwon Lee, CEO of 인터넷 바카라사이트 BioScions

[by Kang, In Hyo] "The multi-specific antibody drug candidate, 'ARK102 (development code),' demonstrated complete response (CR) in preclinical trials, exhibiting markedly enhanced tumor-suppressive efficacy compared to approved antibody-drug conjugates (ADCs)."
In a recent interview with <THE BIO>, Seungwon Lee, CEO of ARKgen BioScions, emphasized the animal study results of ARK102, one of the company’s core pipeline candidates, highlighting its potential as a next-generation anticancer drug.
Founded in July 2021, 인터넷 바카라사이트 BioScions is a biotech venture dedicated to the development of immuno-oncology therapeutics founded on proprietary platform technology. Now in its fifth year, the company is currently developing an immuno-oncology pipeline structured around two pillars: multispecific antibody therapeutics and therapeutic cancer vaccines.
The name 'ARKgen BioScions' combines the elements 'Ark,’ 'Gen' (to create), and 'Scions' (descendants of a distinguished lineage), reflecting the company's commitment to ‘building a new ark in the biopharmaceutical field.’
"I have been researching antibody therapeutics since my days at LG Chem (formerly LG Life Sciences), where my work was firmly rooted in this field. Observing the industry’s transition from monoclonal antibodies to bispecific, tri-specific, and ultimately tetra-specific antibodies, I anticipated that multispecific antibody development would become the future focus. This is the reason I chose to pursue this field," Lee stated.
"Taking into account technological progress and prevailing industry trends, I concluded that multispecific antibodies represent a field not yet fully penetrated by competitors. Even globally, relatively few companies are developing them as a new platform," Lee further commented.
ARKgen BioScions was founded by CEO Seungwon Lee with six co-founders. Lee currently holds approximately 51% of the company’s shares, while the six co-founders collectively own around 16%. All founding members are experts with prior experience at LG Chem.
Lee spent 23 years as head of the LG Chem Research Institute, where he led biopharmaceutical R&D and commercialization efforts. After his departure, he founded the company with like-minded colleagues to pursue his aspiration of developing innovative novel drugs. "Rather than competing against each other, I wanted to embrace new challenges alongside colleagues who shared the same vision," he said. "I was able to start this venture thanks to the support of colleagues who continued to stand by me even after retirement."
The ‘multi-specific antibodies’ under development at ARKgen BioScions represent a more advanced modality than monoclonal antibodies, which bind to a single antigen, or bispecific antibodies, which target two antigens simultaneously.
"Multi-specific antibodies exhibit high selectivity in differentiating cancer cells from normal cells and are capable of binding to multiple targets simultaneously, thereby maximizing anticancer effects. Their binding affinity is strengthened, operating on a principle comparable to having multiple arms." Lee explained.
"Multi-specific antibodies also offer the advantage of addressing the challenge of 'tumor heterogeneity,' a phenomenon in which cancers acquire drug resistance. This is why the global pharmaceutical and biotechnology industries are transitioning from monoclonal antibodies to multispecific antibodies," he added.
According to Lee, cancer is not driven by a single antigen but can express multiple antigens simultaneously. For example, if cancer cells arise by mechanism A and are suppressed with a therapeutic agent, mechanism B may activate immediately, altering the cancer's ‘survival mechanism’ and limiting the effectiveness of a single antibody. In contrast, multispecific antibodies can target several antigens concurrently, allowing them to control these resistant cancer cells.
"Multi-specific antibodies are analogous to antibiotic treatments, in which resistance is reduced through the administration of a combination of agents," Lee explained. "Because novel targets carry substantial risks, we are pursuing the development of multispecific antibodies using targets that are already commercially available and clinically validated."
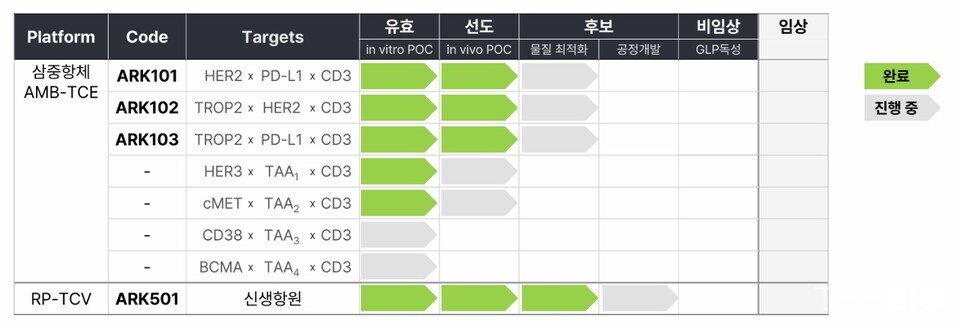
ARKgen BioScions' multispecific antibody platform, designated as ‘AMB (ARKgen Multi-Body),’ uses a proprietary linker to connect antigen-binding sites, enabling the construction of up to penta-specific antibodies. Currently, the company is focusing its efforts on the development of a ‘T-Cell Engager.’
ARK102, the company's flagship pipeline candidate, is a multi-specific antibody candidate engineered to simultaneously target TROP2 (trophoblast cell-surface antigen 2), HER2 (human epidermal growth factor receptor 2), and a CD3 T-cell engager.
ARK102 is being developed primarily for the treatment of HER2-low non-small cell lung 인터넷 바카라사이트 and breast 인터넷 바카라사이트. With its differentiated mechanism of action as a T-cell engager, it is expected to provide a novel treatment option for patients who have developed resistance to existing TROP2/HER2 ADC treatments (Enhertu and Trodelvy).
"In preclinical in vivo studies, ARK102 demonstrated both anticancer efficacy and safety. Complete responce (CR) was observed at all dose levels, with CR also confirmed in the low- dose and mid-dose cohorts," Lee remarked.
"ARK102 demonstrated superior efficacy compared to other ADCs under equivalent conditions. Whereas competing products are formulated for intravenous (IV) administration, ARK102 is being developed as a subcutaneous (SC) formulation, enhancing patient convenience and therapeutic competitiveness," he further emphasized.
ARKgen BioScions intends to develop ARK102 as a once-weekly formulation to minimize toxicity risks. "By prolonging the half-life and regulating exposure time, we can lower the dosage while maintaining therapeutic efficacy. Given the high toxicity potential of T-cell engager drugs, employing a strategy that achieves efficacy at reduced doses is essential," Lee explained.
The company is currently developing a formulation with an extended half-life and plans to proceed with toxicity studies in monkeys as the next step. Lee stated, "If ARK102 successfully passes these evaluations, it will be confirmed as the final development candidate."
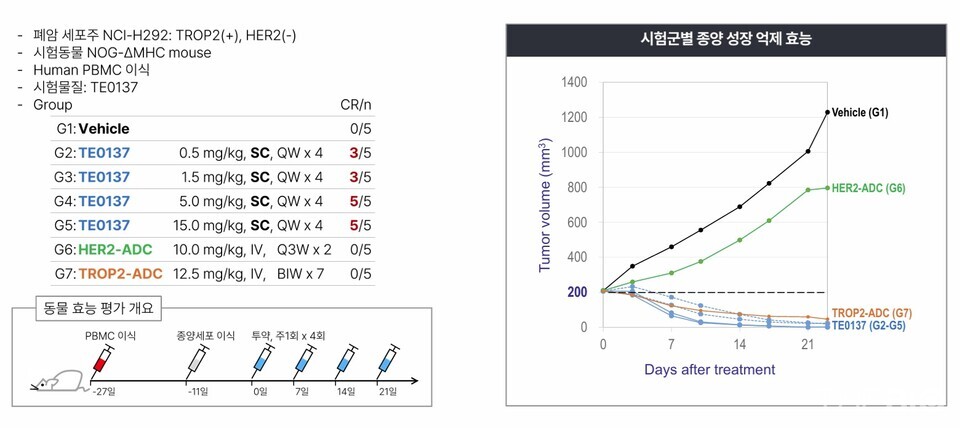
Preclinical animal studies of ARK102 demonstrated CR in all dose groups. "We divided the experimental animals into four groups of five subjects each. In some groups, CR was observed in all animals, while in another, three animals achieved CR," Lee explained. "Efficacy was evident across the full dosing range, from 0.5㎎/㎏ to 15㎎/㎏. This broad therapeutic window, spanning low to high doses, reflects a wide therapeutic window, which serves as an important indicator for reducing the risk of clinical failure."
"The absence of weight change across the dosing range from low to high indicates a reduced risk of toxicity," Lee further noted. "CR was also observed in animals with tumors that had progressed to volumes of 700-900㎣."
In standard practice, anticancer efficacy evaluations begin when tumors reach approximately 100㎣. However, ARKgen BioScions began dosing at 200㎣ and confirmed efficacy under more stringent conditions, according to Lee. "Some animals achieved CR even when tumors had expanded to 700-900㎣. Although such animals are typically excluded, statistical comparisons with the control group still demonstrated significant differences," he explained.
"T-cell engager drugs carry a considerable potential risk of toxicity, yet this animal study indicated a minimized risk," Lee further noted. "Both therapeutic efficacy and weight recovery were observed, demonstrating both effectiveness and safety."
Notably, ARK102 also exhibited anticancer activity in a re-challenge experiment. This type of study involves reimplanting tumors into animals that achieved CR to assess the durability of the anticancer effect and the efficacy of readministration. In practical terms, it entails re-implanting cancer cells after a single treatment to evaluate whether resistance develops or, conversely, whether immune memory is formed to maintain relapse prevention.
"After confirming that tumors did not recur in the 11 mice that achieved a CR in the initial lung cancer model, we re-implanted cancer cells into the same animals. Although tumor growth was observed, the rate of progression was significantly reduced, and in two mice, tumor size actually decreased," Lee explained.
"This phenomenon appears to result from the formation of 'memory T cells' mediated by the T-cell engager mechanism," he further emphasized. "Tumor growth inhibition was observed even in the absence of drug administration. When tumor progression resumed and the drug was retreated, CR was achieved in three of nine mice. This demonstrates that repeated treatment is possible without the development of resistance."
The remaining six mice were re-implanted with breast cancer cells rather than lung cancer, and Lee observed that tumor growth was likewise significantly reduced in this model. He added, "This suggests that immune activation during the initial tumor removal contributed to growth inhibition in other cancers as well."
ARKgen BioScions successfully completed a pre-A funding round of approximately KRW 4 billion (approximately USD 2.8 million) at the end of 2023 and is currently in the process of a Series A round. "We are aiming to close by the end of this year at the earliest, or by the first half of 2026 at the latest," Lee stated. "The scale will exceed that of the previous round."
Lee has set a target for ARKgen BioScions to pursue an IPO in H2 2028. He outlined a mid- to long-term strategy in which the IPO would not rely solely on clinical development milestones but would instead follow the achievement of at least two meaningful business development (BD) outcomes. This is to reinforce investor trust and ensure business sustainability post-IPO.
"We are confident in the strength of our internal pipeline. We regard out-licensing and clinical success as 'a matter of time' rather than of substance," Lee said. “With a solid pipeline and exceptional talent to drive its execution, I am confident that, despite potential challenges, ARKgen BioScions will ultimately provide clear hope to patients suffering from cancer,” he added.

