- From immuno-oncology to ADCs and neurological disorders… Pipeline diversification driven by open innovation
- Featuring NEX-I's 'NXI-101', LigaChem's 'LCB97', and SK Bio바카라사이트 캡틴s' 'cenobamate'
- Accelerating global development and commercialization by addressing OPDIVO LOE while advancing the 'Deciphera' acquisition in parallel
[by Sung, Jae Jun] The Japanese pharmaceutical company Ono Pharmaceutical (hereinafter referred to as Ono) has reaffirmed its global growth strategy centered on ‘immuno-oncology,’ underscoring the outcomes of its technology-driven collaborations with Korean biotechnology companies as a core engine for pipeline expansion. Collaborative assets involving Korean biotech partners, including NEX-I, LigaChem Biosciences (hereinafter referred to as LigaChem), and SK Biopharmaceuticals, were prominently reflected throughout the presentation, illustrating an open innovation-based strategy designed to broaden and strengthen Ono’s pipeline.
During a recent corporate presentation at the 2026 JP Morgan Healthcare Conference held at the Westin St. Francis Hotel in San Francisco, Ono outlined its mid- to long-term R&D strategy centered on oncology, immunology, and neurological diseases, alongside a roadmap for global business expansion. The presentation was delivered by Toichi Takino, President and Chief Operating Officer (COO) of Ono, who detailed the company's pipeline restructuring and global commercialization plans in anticipation of the loss of exclusivity (LOE) for OPDIVO (nivolumab). "There were concerns about the patent expiration of OPDIVO, but we are now seeing positive signs that will support growth in the post-OPDIVO era," said Takino.
◇Pipeline expansion via open innovation, featuring collaborative assets from Korea
바카라사이트 캡틴 underscored its R&D strategy, which combines internally driven novel drug development capabilities with active collaboration across the global pharmaceutical and biotechnology industries, as well as academia, through an open innovation framework. The presentation highlighted multiple pipeline assets acquired through partnerships with Korean companies, presenting them as tangible outcomes that exemplify the effectiveness of this open innovation strategy.
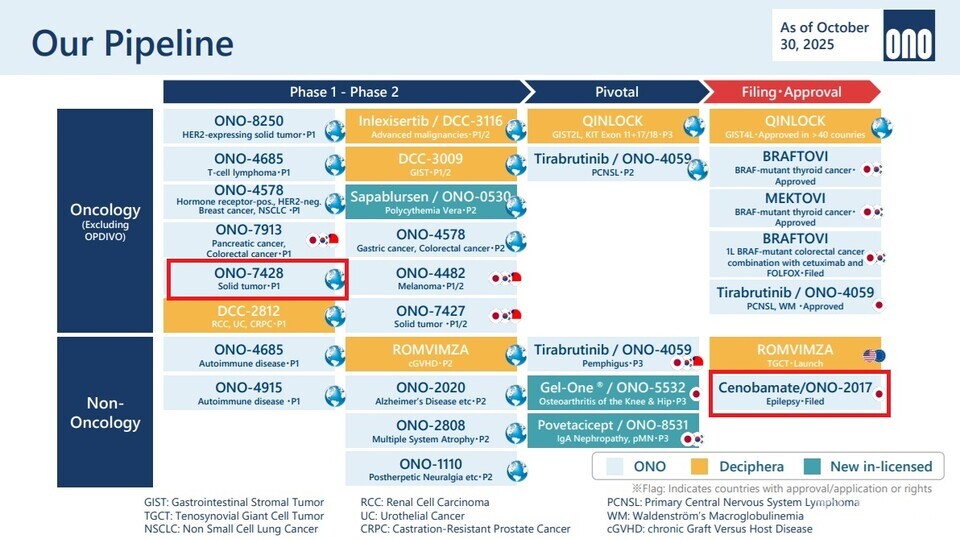
First, ‘NXI-101 (Ono development code ONO-7428),’ an immuno-oncology drug candidate licensed in 2024 from the Korean biotechnology company NEX-I, was introduced as an early-stage clinical asset for the treatment of solid tumors. The candidate is being advanced as one of the follow-up assets intended to reinforce the immuno-oncology portfolio in anticipation of the LOE for OPDIVO, and is currently progressing through early-phase clinical development on a global scale.
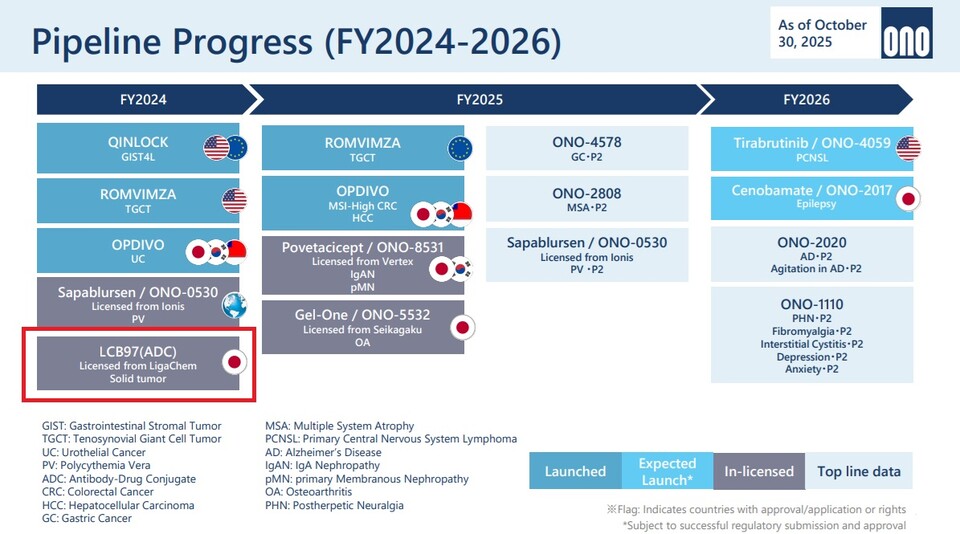
Furthermore, Ono has incorporated ‘LCB97 (development code),’ an antibody-drug conjugate (ADC) candidate obtained through a licensing agreement with the Korean biotechnology company LigaChem, into its development pipeline. LCB97 is classified as an ADC program targeting solid tumors, and Ono highlighted this asset as a representative outcome of its open innovation strategy. Through this example, the company emphasized its commitment to securing global technology, irrespective of geographic boundaries or specific research institutions.
LCB97 was acquired through a licensing and joint research agreement with LigaChem in October 2024. Under the terms of the agreement, 바카라사이트 캡틴 secured global rights for the development and commercialization of LCB97, along with collaboration in the discovery of additional candidates leveraging LigaChem's ADC platform technology. 바카라사이트 캡틴 indicated that it intends to gradually expand its ADC portfolio through such external technology acquisitions.
In the non-oncology sector, SK Biopharmaceuticals' epilepsy treatment cenobamate (development code 바카라사이트 캡틴-2017) was highlighted as a key commercialization asset. The drug was presented as a candidate slated for regulatory approval and market launch in Japan and is included in 바카라사이트 캡틴's mid-term growth roadmap.
During the presentation, Takino stated, "We are aiming to launch cenobamate in Japan in fiscal year 2026, in parallel with the U.S. launch of tirabrutinib," adding, "The commercialization timeline for this asset is now clearly within sight."

◇Post-OPDIVO LOE strategy: Deciphera acquisition accelerates global expansion
Ono also addressed the anticipated impact of the patent expiration of OPDIVO. During a Q&A session, Takino explained, "The transition to a subcutaneous (SC) formulation, the expansion of combination therapies, and the continued clinical progress of our follow-up pipeline are collectively offsetting the effects of the patent expiration." He further cautioned against concerns regarding short-term earnings volatility, emphasizing that OPDIVO's formulation conversion and combination strategy have already been incorporated into the company's current revenue structure.
Takino further emphasized the company’s strengthened global development and commercialization capabilities following the acquisition of the U.S. biopharmaceutical company Deciphera Pharmaceuticals. "Through the acquisition of Deciphera, we have established a system that enables that direct global expansion of our pipeline assets developed in Japan, supported by our clinical development and commercialization infrastructure in the United States and Europe. Our strategy is to seamlessly connect research outcomes generated in Osaka with global business execution," he further commented.
Deciphera possesses an oncology pipeline centered on kinase inhibitors, including QINLOCK (ripretinib), a treatment for gastrointestinal stromal tumors (GIST). Ono acquired Decipher in 2024 for around USD 2.4 billion (approximately KRW 3.54 trillion), thereby securing in-house clinical development and commercialization capabilities in the U.S. and Europe. This acquisition is widely regarded as a strategic foundation for accelerating the global expansion of Ono’s Japan-originated pipeline developed into international markets.
Following the acquisition of Deciphera, Ono has advanced the global commercialization of its oncology portfolio, including QINLOCK and ROMVIMZA, a treatment for synovial giant cell tumor (TGCT). In parallel, the company disclosed new clinical data from its mid- to late-stage pipeline, including ONO-4578 (development code), ONO-2808 (development code), and Sapablursen (development code ONO-0530). Commenting on these developments, Takino said, "We are currently observing positive signals across the entire pipeline." He added, "We anticipate seeing a substantial volume of clinically meaningful data over the next one to two years."
◇Sustaining global technology adoption policies while expanding strategic investment
바카라사이트 캡틴 plans to continue to pursue strategic investments (SI) and pipeline acquisitions. Over the past three years, the company has deployed about JPY 500 billion (approximately USD 3.1 billion) towards mergers and acquisitions (M&A) and the in-licensing of pipeline assets, and it currently retains additional investment capacity of roughly JPY 100 billion.
In this context, 바카라사이트 캡틴 explained that, in evaluating potential investment targets, it places greater emphasis on scientific feasibility, technological differentiation, and global scalability than on the specific stage of development. The company further noted that it adopts a broad evaluation framework encompassing assets from early research through late-stage development and commercialization, with strategic synergy with its internal R&D capabilities serving as a central selection criterion.
“Ono does not confine its investment activities to specific countries or technology areas,” said Takino. “We are prepared to actively evaluate even early-stage assets, provided they demonstrate scientific significance and align with our long-term strategic objectives,” he added.

