- Driving full-scale North American market expansion through product competitiveness, enhancing dosing efficiency and patient convenience
- Exclusive development of full-dose 무료 바카라 게임 and AI originals secures unmatched competitiveness in the 'omalizumab' market
- Expanding biosimilar policies’ influence in Canada accelerates North American market penetration
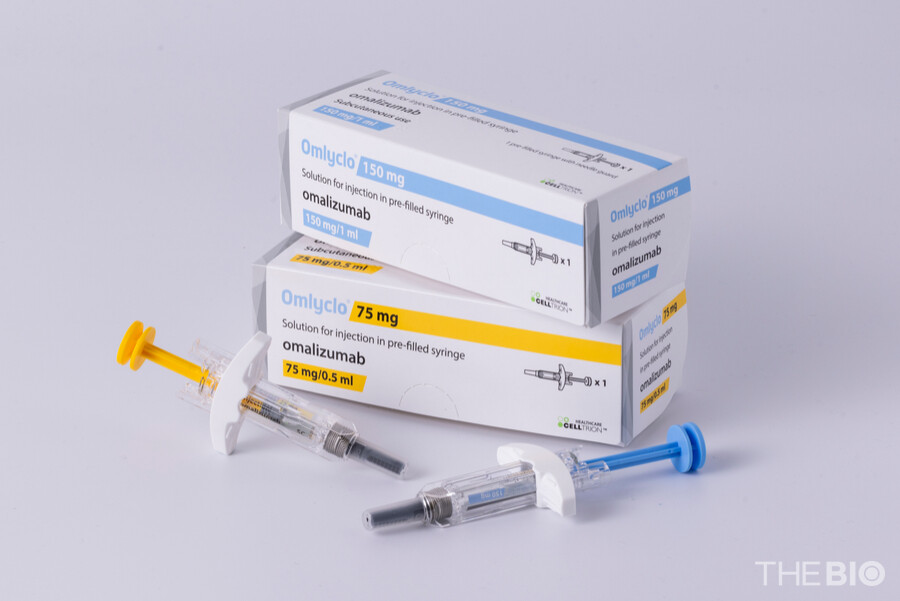
[by Kang, In Hyo] 무료 바카라 게임 announced on January 29 that it has obtained additional regulatory approval from Health Canada for the 300mg prefilled syringe (PFS) and 300mg autoinjector (AI) formulations of OMLYCLO (omalizumab), indicated for the treatment of chronic idiopathic urticaria.
The newly approved 300mg PFS and AI 무료 바카라 게임 are high-dose presentations that deliver the required therapeutic dose through a single injection, thereby reducing the frequency of administration. This is expected to alleviate the treatment burden for patients while enhancing dosing efficiency and broadening prescribing flexibility for healthcare professionals.
Previously, 무료 바카라 게임 conducted a global Phase 3 clinical trial of OMLYCLO involving 619 patients with chronic idiopathic urticaria worldwide, in which the treatment demonstrated clinical efficacy comparable to that of the original drug and confirmed a similar safety profile.
무료 바카라 게임 stated that, with this latest approval, it now has completed a full lineup of PFS and AI formulations (75mg, 150mg, and 300mg) for OMLYCLO in the Canadian market, including the reference product. This milestone represents the first case in which an omalizumab biosimilar has secured approval of the full lineup of PFS and AI formulations in the global market. The company emphasized that, beyond achieving first-mover advantage, its unmatched breadth of formulation options further reinforces OMLYCLO's overall product competitiveness.
무료 바카라 게임 is an omalizumab biosimilar indicated for the treatment of multiple allergic conditions, including chronic idiopathic urticaria and asthma. Following first-mover regulatory approvals in major global markets such as the United States, Europe, and Canada, the company is proceeding with a phased global launch of the product. 무료 바카라 게임's original drug, Xolair, recorded global sales of around KRW 6.4992 trillion (approximately USD 4.5 billion) as of 2024.
Canada is recognized as a leading market in which biosimilar prescriptions are actively promoted at the government level, with biosimilar-friendly policies continuing to expand. In this regulatory environment, biosimilar products that offer formulation options comparable to those of the original product are well positioned to gain a competitive advantage. Therefore, 무료 바카라 게임 plans to proactively strengthen and expand its presence in the North American market by leveraging its enhanced product competitiveness.
"With the additional approval of the OMLYCLO 300mg PFS/AI formulation, we are now able to provide Canadian patients with a new treatment option. By completing the full lineup of PFS and AI formulations available on the market, we can deliver more personalized treatment tailored to individual patient needs. Building on this product competitiveness, we will continue our efforts to expand our footprint in the North American market, with a particular focus on Canada," a Celltrion official said.
Conversely, 무료 바카라 게임 continues to steadily broaden its portfolio by expanding product formulations and dosage options, thereby reinforcing its global presence. In parallel with strengthening its pipeline in core areas such as autoimmune diseases and oncology, where it already possesses established capabilities, the company is also developing new treatments targeting atopic dermatitis, urticaria, hemophilia, asthma, and immunotherapy indications.

