- [Interview] Lee Chae-joon, Co-CEO of Ildong 바카라사이트 굿모닝
- Ildong 바카라사이트 굿모닝 Group showcases key achievements at the JP Morgan Healthcare Conference
- "Small-molecule oral GLP-1 candidate set to enter U.S. Phase 2 clinical trial in H2 2026"
- "Global partnership for P-CAB candidate 'padoprazan' expected to be finalized within the year"
- "Idience's PARP inhibitor draws attention as a dual payload for ADCs"
- "Ildong 바카라사이트 굿모닝 to validate R&D value through tangible results"

[by Ji, Yong Jun] The global ‘obesity’ therapeutics market, long dominated by multinational pharmaceutical companies Eli Lilly and Novo Nordisk, is undergoing notable transformation as new entrants gain momentum. Among Korean firms, Ildong Pharmaceutical is emerging as a potential ‘ready game changer.’ Notably, the company held meetings with more than 50 global companies at this year's JP Morgan Healthcare Conference, signaling tangible progress toward full-scale partnership negotiations. In contrast to last year's exploratory discussions with multinational companies at the conference, this year's engagements are expected to advance into a ‘full-scale negotiation phase,’ marked by in-depth discussions with a wide range of potential partners.
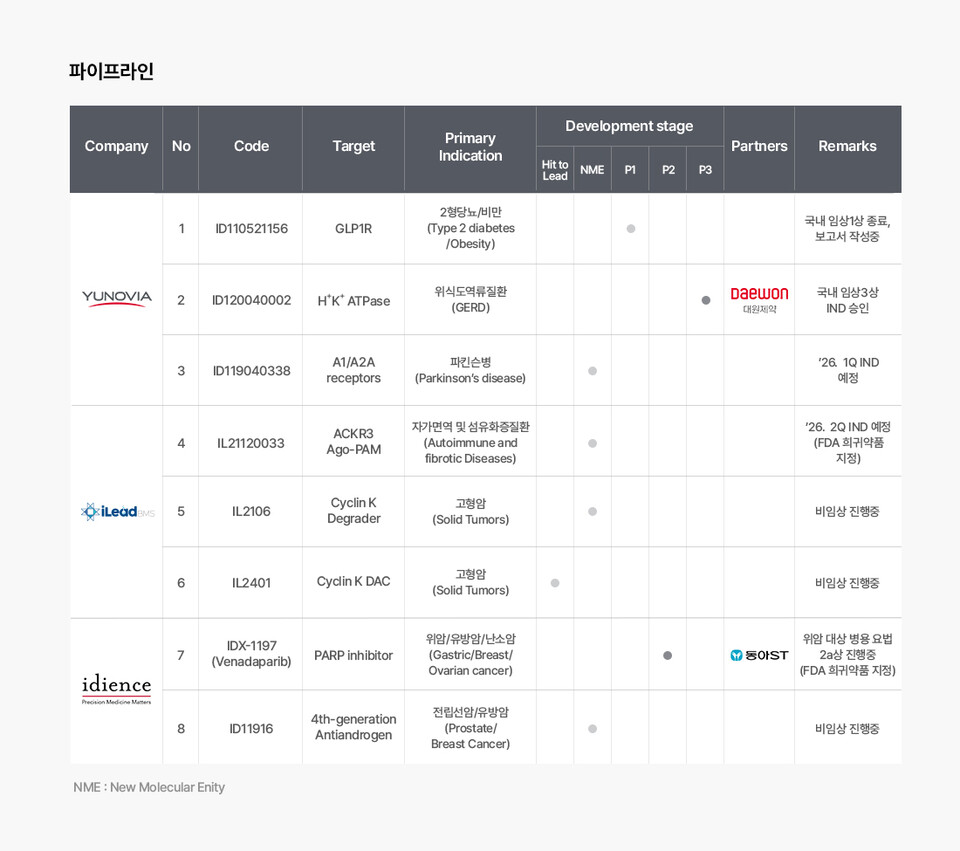
Central to Ildong Pharmaceutical's ongoing negotiations is its small-molecule, orally administered glucagon-like peptide 1 (GLP-1) receptor agonist (RA) obesity drug candidate, ‘ID110521156 (development code).’ In parallel, momentum is building around global partnership efforts for padoprazan, a potassium-competitive acid blocker (P-CAB) under development for the treatment of gastroesophageal reflux disease, while the potential expansion of Idience's PARP inhibitor into an antibody-drug conjugate (ADC) dual payload strategy is also drawing attention. These developments are viewed as forming Indong Pharmaceutical’s R&D ‘triangular formation,’ reinforcing the company’s growing presence in the global market.
In a recent interview with <THE BIO>, Lee Chae-joon, Co-CEO and President of Ildong Pharmaceutical, remarked, "This year's JP Morgan Healthcare Conference served as an opportunity to reaffirm the company’s positioning amid the rapidly evolving competitive landscape of the obesity treatment market. The on-site meetings provided us valuable 'hints' and, more importantly, clarified the 'next step' for the company’s future became." Inquiries and discussions concerning confidentiality agreements (CDAs) and due diligence related to Ildong Pharmaceutical's GLP-1RA candidate are actively underway, further heightening expectations for concrete progress within the year.
This year, the R&D affiliates of the Ildong 바카라사이트 굿모닝 Group, including its novel drug development subsidiary Yunovia, oncology-focused company Idience, and new substance discovery firm iLeadBMS, convened at the JP Morgan Healthcare Conference held in San Francisco from January 12 to 15 to pursue global business development (BD) opportunities. During the conference, Yun Paul Woong-sup, Co-CEO (Chairman) of Ildong 바카라사이트 굿모닝, was frequently observed attending investor relations (IR) presentations by global 바카라사이트 굿모닝 companies with obesity pipelines, while Co-CEO Lee Chae-joon focused on BD activities, participating in approximately 50 consecutive meetings throughout the event.
What Ildong Pharmaceutical observed at the JP Morgan Healthcare Conference was a clear shift in the dynamics of the global obesity treatment market. "The market has completely changed compared to a year ago. Alongside the established powerhouses like Eli Lilly and Novo Nordisk, a growing number of biotechnology companies have entered the battle for dominance in the obesity market,” Lee commented. “'Weight loss' had been the definitive criterion until last year, the focus has now shifted toward 'therapies that can be administered safely for 10 or 20 years or even a lifetime, without compromising patients’ quality of life,” he added. According to Lee, Ildong Pharmaceutical's ID110521156 is well aligned with the evolving market needs.
◇Oral GLP-1 candidate ID110521156 gains global attention for zero liver toxicity, with U.S. Phase 2 entry planned for H2
Another notable change is that, following the disclosure of Phase 1 clinical trial results for ID110521156 last year, the perception among major 바카라사이트 굿모닝 companies has moved from initial skepticism to growing confidence. Until last year, the absence of clinical data prompted a cautious and largely reserved response, but the biggest change this year has been the availability of concrete, quantitative data.
"In the Phase 1 clinical trial of ID110521156, we confirmed a body weight loss effect of up to 13.8% within just four weeks of dosing. Of particular importance, our partners took note of the absence of any changes in liver toxicity indicators (ALT/AST)," Lee remarked. Unlike Orfoglipron, the competing candidate developed by Eli Lilly, which has a prolonged half-life and raised concerns about potential accumulation in the body, ID110521156 is cleared within 24 hours and is suitable for once-daily administration, factors that clearly differentiate the compound.
On the basis of this growing confidence, Ildong Pharmaceutical announced plans to initiate Phase 2 clinical trials in the United States in the second half of this year. “While the Korean clinical trial was conducted in a population with an average body mass index (BMI) of 28, the U.S. Phase 2 clinical study will seek to confirm that the drug’s efficacy can be maximized in patients with severe obesity with a BMI of 30 or higher. We plan to streamline the otherwise complex dose-adjustment (titration) process to ensure both safety and patient convenience,” Lee expressed.

◇Regional partnerships for P-CAB 'padoprazan' come into focus, as PARP inhibitors emerge as dual-payload ADCs
The company has further clarified its BD strategy for 'padoprazan,' another core asset within its P-CAB family. Rather than pursuing partnerships with top-tier multinational 바카라사이트 굿모닝 companies that already possess well-established gastrointestinal portfolios, Ildong 바카라사이트 굿모닝 will prioritize regional second-tier players with a more immediate need for new product introductions.
"We have identified influential partners across Latin America, Southeast Asia, the Middle East, and China. We are currently engaged in positive discussions with the most promising partners and expect favorable outcomes within the year," Lee said. Padoprazan's patent protection, which extends through 2041, provides a strong foundation for global business development activities, including technology transfer (L/O) opportunities.
Idience's PARP inhibitor has recently attracted renewed attention in the ADC field, which has emerged as a hot topic in the biotechnology industry. Lee explained that the compound’s distinctive chemical structure renders it particularly well suited for applications as dual-payload ADCs.
"Interest in Idience's PARP inhibitors has increased markedly since they were assessed as suitable candidates for use as 'dual-payload' ADCs. Even within the global PARP inhibitor landscape, there have been responses noting that 'they have a unique structural profile, and their chemical structure enables clean binding and efficient disassembly,'" Lee stated.
He further continued, stating, "Some antibody and linker developers have indicated that Idience's compound constitutes a technological solution, given the absence of PARP inhibitors that satisfy the structural requirements for ADC dual payloads. Since then, related discussions have advanced at a rapid pace."
◇“Seven years of focused R&D investment mark a period of endurance and commitment, with results expected this year through a ‘selection and focus’ R&D strategy”
Having validated the strategic direction of its R&D portfolio at this year's JP Morgan Healthcare Conference, Ildong 바카라사이트 굿모닝 is now concentrating on translating this momentum into concrete outcomes. Moving beyond the phase of demonstrating potential, the company is determined to make this year a turning point in which its R&D investments bear fruit.
“Over the past five to seven years, we have endured a period of quiet perseverance and sustained investment, during which Ildong Pharmaceutical has fundamentally transformed itself from a conventional pharmaceutical company into a global, R&D-driven new drug developer,” Lee stated. “Although these bold R&D investments at times imposed a financial burden, this year’s JP Morgan Healthcare Conference demonstrated on a global stage that our direction was indeed the right one,” he added.
“This year, we will maximize efficiency through an R&D strategy of ‘selection and concentration’ and demonstrate the value of Ildong Pharmaceutical’s R&D through concrete and tangible results,” Lee further emphasized.

