Atrophic scar regeneration efficacy fully demonstrated, aiming to surpass existing 바카라사이트 정보 limitations through proprietary organoid technology

[by Ji, Yong Jun] 바카라사이트 정보, a company specializing in organoid-based regenerative therapies, announced on January 27 that it has received approval from the Ministry of Food and Drug Safety (MFDS) for its Phase 2 clinical trial plan (IND) for TRTP-101, a treatment candidate for atrophic scar regeneration.
Previously, 바카라사이트 정보 demonstrated excellent tolerability and safety profiles in a Phase 1 clinical trial, while also achieving proof of concept (PoC) by showing meaningful regenerative efficacy, including a 26.6% reduction in scar volume in patients with atrophic scars.
In the forthcoming Phase 2 clinical study, 바카라사이트 정보 intends to further assess the therapeutic efficacy of TRTP-101 in patients with atrophic scars. Atrophic scars arise from collagen loss within the dermal layer and, while they can substantially impair patients' quality of life and psychological well-being, there remains a lack of fundamental regenerative treatment options.
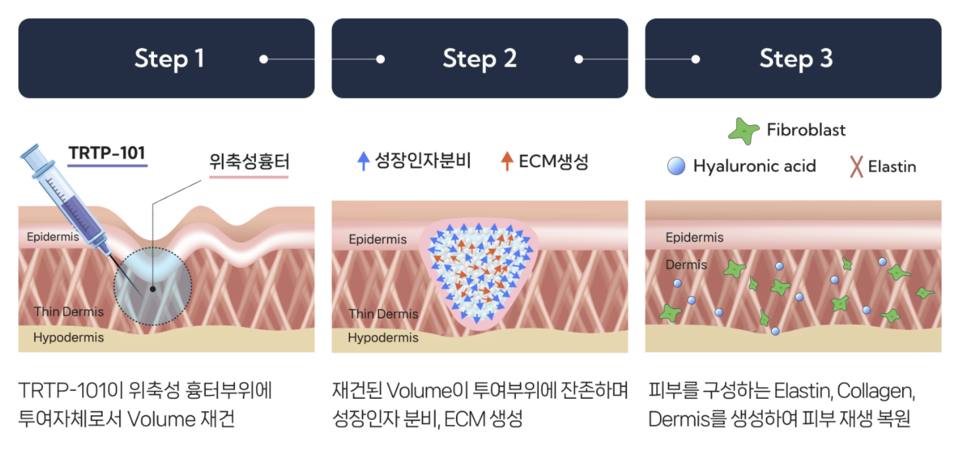
바카라사이트 정보101 is a next-generation organoid-based regenerative therapy designed to induce structural restoration of skin tissue, and it is therefore regarded as a promising innovative alternative with the potential to overcome the limitations of existing treatments.
In 2025, 바카라사이트 정보101 was selected for a regenerative medicine treatment and treatment technology development program led by the Korean Fund for Regenerative Medicine, receiving national-level support. This large-scale government R&D initiative, backed by a total investment of KRW 595.5 billion (approximately USD 417 million) through 2030, provides support across the full regenerative medicine lifecycle, encompassing basic research, clinical development, and eventual commercialization.
바카라사이트 정보' current initiative is centered on facilitating the regulatory approval and practical implementation of clinical trials for regenerative medicine therapies. According to the company, this effort extends beyond the conduct of Phase 2 clinical trials to include the advancement of chemistry, manufacturing, and controls (CMC), a critical source of competitiveness for organoid-based treatments, as well as the establishment of scalable mass-production processes.
In parallel with TRTP-101, 바카라사이트 정보 is advancing research and development of an organoid-based cartilage regeneration treatment (TRTP-201). This program has been selected for support under an inter-ministerial task force and is currently preparing to seek Investigational New Drug (IND) approval from the MFDS.
"Approval of the Phase 2 IND marks a pivotal milestone, demonstrating that CELLinCELLS' organoid regenerative technology has progressed beyond the research stage and entered a phase of full-scale commercialization,” said Cho Jae-jin, CEO of CELLinCELLS. “By successfully completing this clinical trial, we aim to deliver a new treatment option for patients with atrophic scars and to leap forward as a leading player in the global regenerative medicine market," he added.

