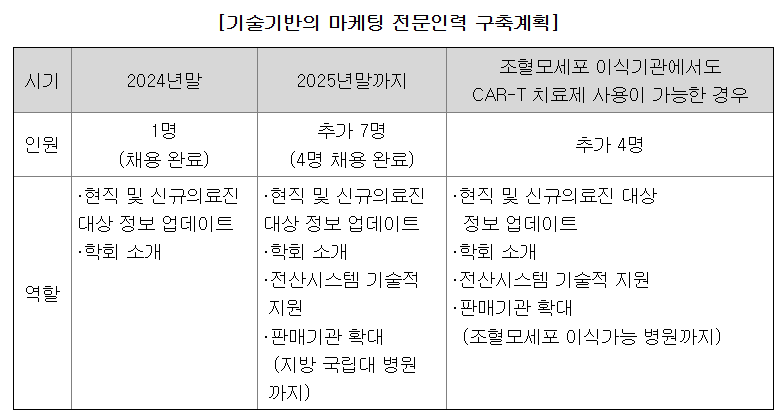
- Focused approach on select medical professionals... Seven marketing specialists to be added
- Integrated management system for hospitals and GMP facilities established to enhance precision and efficiency
- Competition heats up as YESCARTA gains domestic approval, overtaking KYMRIAH
- 실시간-바카라사이트 highlights the competitive advantage of domestic manufacturing with high complete remission rates
- Research team expanded to 111 members for 실시간-바카라사이트 indications... Phase 2 clinical trial in adult ALL approaching
[by Yu, Suin] Curocell's next-generation chimeric antigen receptor T-cell (CAR-T) therapy, RIMQARTO (anbal-cel, development code: CRC01), is already facing mounting competition ahead of approval, following the recent approval of the U.S. Gilead Sciences’ YESCARTA (axicabtagene ciloleucel) as the third CAR-T treatment available in Korea. In response, Curocell is ramping up preparations for market entry, expanding its marketing and R&D teams ahead of RIMQARTO's commercialization. The company aims to carve out market share by leveraging a differentiated marketing strategy and the clinical strengths of RIMQARTO.
◇Securing ‘technology-driven marketing talent’…Six recruits finalized by Q3
실시간-바카라사이트 announced on August 18 that it has been steadily building out its marketing team, bringing on four professionals in the first half of this year, two in Q1 and two in Q2. Recruitment continued into Q3, raising the headcount to six, with plans to expand the team to seven by year-end.
The newly recruited staff will take on responsibilities ranging from updating information for existing and prospective medical professionals to engaging with medical societies, providing IT system support, and expanding sales channels into regional and national university hospitals. Unlike most companies, which typically establish separate sales organizations for product distribution, Curocell has built a ‘technology-driven marketing team’ designed to communicate effectively the value of RIMQARTO directly to a select group of specialized medical professionals, reflecting the unique nature of CAR-T therapy.
CAR-T therapy is a personalized treatment derived from the blood of terminal cancer patients, with distribution channels limited to major general and regional hospitals that handle severe oncology cases. To strengthen its position in this specialized market, 실시간-바카라사이트 last year appointed Senior Director Lee Seung-won, a seasoned marketing expert in blood cancer and CAR-T therapy. Lee previously spearheaded the launch of KYMRIAH (tisagenlecleucel), the first CAR-T therapy introduced in Korea.
Curocell is also preparing to expand its workforce further, with plans to hire four additional staff if CAR-T therapy becomes available at hematopoietic stem cell transplant institutions. The move follows revisions to the Act on Safety and Support of Advanced Regenerative Medical and Advanced Biopharmaceuticals (Advanced Biopharmaceuticals Act), which eased the requirements for designating ‘advanced regenerative medicine institutions’ authorized to provide CAR-T therapy. In practice, the collection of T cells from patient blood closely resembles the hematopoietic stem cell transplantation process, meaning the infrastructure for CAR-T treatment can be operated at a comparable level.
RIMQARTO is currently under review by the Ministry of Food and Drug Safety (MFDS) for the indication of ‘relapsed or refractory diffuse large B-cell lymphoma (DLBCL).’ "We have set our sights on securing approval within the year for RIMQARTO. Even ahead of formal approval, we are working closely with medical institutions on commercial sales preparations, so we are expanding our marketing team and plan to continue to do so," Curocell stated.
◇Competitor drug 'YESCARTA' approved for launch in H1 2026…"RIMQARTO boasts superior 'complete remission' and strong competitiveness"
Even if RIMQARTO receives MFDS approval, it will face challenges as a latecomer in the DLBCL treatment market. KYMRIAH, introduced in Korea in 2021, has already established market presence with reimbursement coverage. Janssen followed with 'CARVYKTI (ciltacabtagene autoleucel)' in 2023, and most recently, Gilead's 'YESCARTA' secured approval as the third 실시간-바카라사이트 therapy in Korea.
However, CARVYKTI has yet to be launched more than two years after approval, while YESCARTA's market penetration will hinge on whether it secures reimbursement. Both therapies are already commercially available in global markets, but YESCARTA holds a competitive advantage in Korea, as it has been approved for use as a second-line treatment.
In Korea, DLBCL accounts for an estimated 6,000 new cases each year. Demand for 실시간-바카라사이트 therapy in second-line settings is especially strong, as roughly 40% of patients relapse after first-line treatment and many fail to respond to existing treatments. Against this backdrop, industry observers see YESCARTA approval as highly significant, marking it as the only 실시간-바카라사이트 option currently available for second-line treatment in Korea.
YESCARTA has also received approval for use in adult patients with relapsed or refractory diffuse large B-cell lymphoma and primary mediastinal B-cell lymphoma (PMBCL) after second-line or later systemic 실시간-바카라사이트, an area where no other treatments are currently approved in Korea. YESCARTA is expected to launch in Korea in the first half of 2026.

Nevertheless, Curocell argues that RIMQARTO holds competitive advantages over rival drugs in supply timelines, efficacy, and safety. Its biggest strength lies in being manufactured at the company’s GMP facility in Daejeon, which significantly reduces turnaround time. Unlike KYMRIAH and YESCARTA, which require patient blood to be shipped to the U.S. for manufacturing and then re-imported to Korea, a process that typically takes one to two months, RIMQARTO can be produced domestically, streamlining the sequence of CAR-T therapies production, from immune cell collection to manufacturing, quality testing, and administration.
Curocell has also developed ‘CUROLINK,’ an integrated platform that enables real-time tracking and management of the entire process, from prescription to administration. Designed with input from frontline treatment sites, the system is intended to streamline hospital workflows. With CAR-T therapies involving highly complex manufacturing, logistics, and administration steps, the company sees precise scheduling and supply chain efficiency as critical advantages.
Because 실시간-바카라사이트 therapy is produced and transported on a patient-by-patient basis, treatment must be closely coordinated with hospitals, patient dosing schedules, and product shipment schedules. Once a dosing date is set, patients are typically admitted four to five days in advance to undergo three days of lymphodepleting therapy. The 실시간-바카라사이트 product, delivered in frozen form on the scheduled date, is then administered, followed by a one- to two-week monitoring period for potential side effects.
"From the moment a prescription is issued, an 'integrated management system' is needed to coordinate scheduling between hospitals and GMP facilities, ensuring patient safety and precise timing," Curocell explained. "The company added that it plans to roll out its CUROLINK platform to major hospitals in Korea in line with RIMQARTO’s launch and will deploy marketing specialists to provide on-site support, minimizing inconvenience to medical staff."
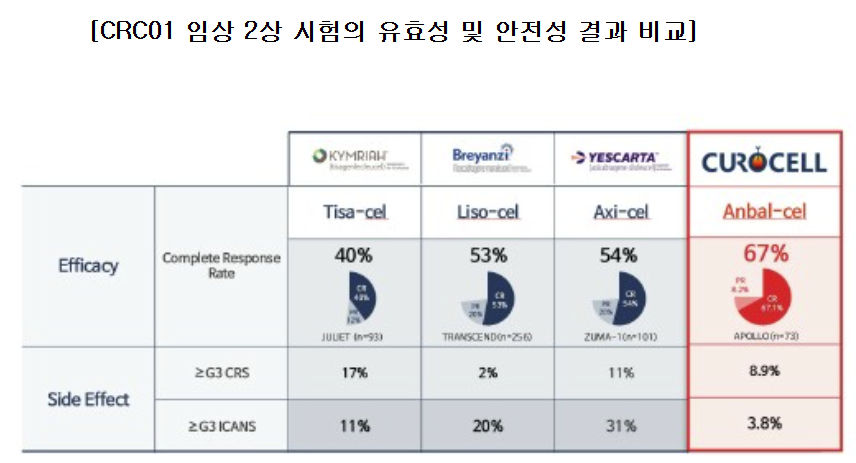
The company also asserts that RIMQARTO holds an efficacy advantage. While YESCARTA demonstrated a complete remission (CR) rate of 54% of patients participating in clinical trials, indicating the complete disappearance of cancer cells, 실시간-바카라사이트 reports that RIMQARTO achieved a CR rate of 67% of patients.
The global 실시간-바카라사이트 therapy market is undergoing a shift as new products demonstrate stronger clinical efficacy compared to existing treatments. Since the arrival of YESCARTA, sales of KYMRIAH (CR 40%) have steadily declined.
YESCARTA, however, has seen a slight dip in sales amid increasing market fragmentation. The arrival of Bristol-Myers Squibb's Breyanzi (lisocaptagene maraleucel, CR 53%), which entered the market later, did not differ significantly. As a result, YESCARTA's global sales slipped 5% year-on-year in Q2, totaling USD 393 million (approximately KRW 550 billion).
"RIMQARTO has achieved a complete remission rate of 67%, outpacing YESCARTA or Breyanzi. Because insurance coverage allows only a single dose for terminal cancer patients, we believe RIMQARTO's competitive efficacy will translate into lasting competitiveness in the market," Curocell emphasized. "While acknowledging the potential emergence of rival modalities such as bispecific antibodies, Curocell's domestic manufacturing base provides a key advantage in ensuring faster supply."
◇R&D team expansion to broaden indications…Securing Phase 1 clinical trial data in challenging adult ALL trial
Curocell is also pushing to broaden RIMQARTO's indications as part of its strategy to strengthen competitiveness. The company is targeting new indications to include adult acute lymphoblastic leukemia (ALL), systemic lupus erythematosus (SLE), and solid tumors. To support this effort, Curocell has grown its R&D team from 102 at the end of 2024 to 111 by mid-2025. "We are currently working to expand RIMQARTO’s indications, develop a CAR-T pipeline for solid tumors, and internalize viral vector production technology. To achieve this, we require additional research capacity, which is why we are actively recruiting," a Curocell official said.
실시간-바카라사이트 recently filed an amendment to its Investigational New Drug (IND) application with the Ministry of Food and Drug Safety (MFDS) to begin a Phase 2 clinical trial for the treatment of adult ALL. Adult ALL is a rare condition in Korea, with only 200-300 cases each year, and carries a poor prognosis, with a five-year disease-free survival rate of just 35%, significantly worse than in pediatric patients. At present, no CAR-T therapies are approved for adult ALL in Korea, and the rarity and complexity of the disease make clinical trials particularly challenging.
However, 실시간-바카라사이트 noted that Phase 1 results for RIMQARTO not only confirmed safety and early signs of efficacy but also helped establish the optimal dose for advancing into Phase 2. Under the amended IND, the patient population has been expanded and new efficacy evaluation measures introduced, with overall complete remission rate (OCR) designated as the primary endpoint. Additional analyses will include time to response (TTR), duration of remission (DOR), and relapse-free survival (RFS).
Curocell plans to share more detailed Phase 1 clinical trial results at a scientific conference later this year, though the company has not disclosed a specific presentation timeline. "We believe RIMQARTO has the potential to become a new treatment option once it progresses into Phase 2 clinical trials," Curocell stated.

